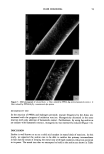
40 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 70 60 50 10 60 80 100 120 140 160 0.8 0.6 0.4 0.2 70 60 50 10 60 80 100 120 140 160 0.6 0.4 0.2 o x • 70 60 50 10 60 80 100 120 140 160 o ._ x 9o Fraction No. Figure 1. Elution profile from Sephadex G-50. A) Protein hydrolysate solution B) Heat-extracted hydrol- ysate from bleached/waved hair C) High-salt-extracted hydrolysate from bleach/waved hair. Protein ex- traction and detection as described in Materials and Methods. Fluorescamine-reactive amino groups (© -- ©) and hydroxyproline (& - &) were monitored as described. Arrows ( •' ) in Part C indicate location of fractions with low levels of hydroxyproline.
COLLAGEN PEPTIDE SUBSTANTIVITY TO HAIR 41 column fractions assayed for hydroxyproline. The results indicate that throughout most of the MW range the hydroxyproline level parallels the relative fluorescence however, in the low MW range (1,000-2,000), where the concentration of fluorescent detectable peptides decreases, the concentration of hydroxyproline-containing peptides actually increases. The highest level of hydroxyproline-containing peptides chromatograph in a range where the fluorescamine-reactive peptides are low in MW and relatively low in concentration. In preliminary experiments, we rechromatographed the 5 % (wt/vol) solution of collagen protein hydrolysate that had been incubated with 3 g of hair tress. The results indicate a diminution of fluorescence-reactive peptides in the 1,000-3,000-MW range, sug- gesting a selective removal of these peptides but not a complete removal of the collagen peptide(s) from this MW range, since only 3-g hair tresses were used in the substanti- vity experiments (data not shown). We have demonstrated that hydroxyproline is present in both the high-temperature and high-salt soaking fractions from hair tresses treated with collagen peptides (see Table II). Accordingly, it was of interest to determine the MW distribution of both fractions of removed peptides relative to the whole cosmetic grade collagen protein hydrolysate. As shown in Figure lB, fluorescamine-reactive peptides in the high-temperature-removed fraction of damaged hair chromatographed in a relatively sharp peak near 2,000-3,000 MW. To identify where the collagen peptides chromatographed, we hydrolyzed the peptides in select column fractions and assayed for the presence of hydroxyproline. The highest concentration of hydroxyproline-containing peptides was present at a slightly lower MW (i.e., 1,000) than the peak of fluorescamine-reactive peptides (3,000). This suggests that there may be different peptides in the collagen hydrolysate, with widely varying levels of hydroxyproline. Furthermore, it is generally known that proline and hydroxyproline as secondary amines escape detection with fluorescamine under certain reaction conditions (12, 13). However, the presence of hydroxyproline in the peptides fractionated on the G-50 column demonstrates that the high-temperature-removed peptides were derived from the collagen hydrolysate applied to the hair. The results in Figure lB demonstrate that there are at least two broad classes of peptides that bind to bleached/waved hair: one containing low levels of hydroxyproline and an- other that doesn't react well with fluorescamine but is relatively high in hydroxyproline content. Additional evidence that the majority of the 1ow-MW hydroxyproline-con- taining peptides and fluorescamine-labeled peaks are derived from collagen comes from our observations that very little fluorescamine-reactive amino groups (2.5%, see Table I) are removed from bleached/waved hair tresses that never saw the collagen hydrolysate. The selective removal and subsequent binding of 1ow-MW peptides (1,000-3,000 daltons) from a complex mixture of cosmetic grade collagen peptides suggests that MW is an important characteristic for peptide binding to bleached/waved hair. Furthermore, control bleached/waved hair tresses (not treated with the protein hydrolysate) soaked and chromatographed on a G-50 column have a fluorescamine-reactive peak in this 1ow-MW range (1,000-3,000 daltons) that comprises less than 5% of the peak RF of the peptides removed by high temperature from bleached/waved hair. In separate experiments, the high-salt-extracted peptides removed from damaged hair
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)