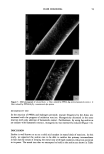
38 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS metic grade collagen hydrolysate (X-250) (washed to remove unbound non-specific peptides) and subsequently treated at high temperature and high salt to remove specific bound peptides. Quantitative and qualitative analyses of fluorescamine-reactive amino groups released from hair tresses in both the high-temperature and high-salt fractions were determined. It should be noted that throughout these experiments we did not change the sequence of peptide soaking (i.e., high salt followed by high temperature). As shown in Table I, relatively low levels of fluorescamine-reactive amino groups were released by high temperature and high salt from both virgin and bleached/waved hair that had never seen the collagen peptides hydrolysate (5% wt/vol). In contrast, in hair tresses (virgin and bleached/waved) treated with collagen peptides, we observed a 10-50-fold higher level of removed fluorescamine-reactive amino groups per weight of hair (3 g) in both the high-temperature and high-salt soaked fractions. These results suggest that most of the fluorescamine-reactive material removed from hair (i.e., both virgin and bleached/waved) treated with the cosmetic protein hydrolysate was due to the release of collagen peptides. Furthermore, in both the high-temperature and high- salt soakings, we observed twofold more fluorescamine-reactive amino groups released from bleached/waved hair than from virgin hair in tresses treated with the collagen hydrolysate. These results are consistent with previous observations that less collagen peptide is bound to virgin than bleached/waved hair as determined by quantitative hydroxyproline analysis of intact hair samples (1-3). Because it was not possible to radiolabel individual peptides in the protein hydrolysate prior to incubation with the hair tresses, we were unable to accurately quantirate the amount of peptide removed from the 5% protein/peptide solution or the exact amount of peptide removed from the hair during both soaking methods. Accordingly, compara- tive hydroxyproline analyses of the various fractions were used as a qualitative determi- nation for the confirmation that collagen peptides from the protein hydrolysate were bound and subsequently removed from the hair tresses. HYDROXYPROLINE ASSAY OF REMOVED PEPTIDES To further demonstrate that the fluorescamine-reactive peptides removed from hair by Table I Fluorescent Quantitation of Amino Groups in the Removed Fractions From Hair Tresses Total RF/Wt Removed fraction hair tress High-temperature removal Virgin (- protein hydrolysate) Virgin (q- protein hydrolysate) Bleached/waved (- protein hydrolysate) Bleached/waved (q- protein hydrolysate) 0.5 M NaCI removal Virgin (- protein hydrolysate) Virgin (q- protein hydrolysate) Bleached/waved (- protein hydrolysate) Bleached/waved (q- protein hydrolysate) O.O92 2.O3O 0.078 5.39O 0.097 1.090 0.090 3.47O RF ---- relative fluorescence. Each measurement represents an average of two hair tresses. All fluorescent measurements were done in duplicate. Each hair tress weighed at least 3.0 grams.
COLLAGEN PEPTIDE SUBSTANTIVITY TO HAIR 39 both high temperature and high salt were derived from collagen, aliquots of each frac- tion were hydrolyzed and assayed for hydroxyproline. As shown in Table II, no hy- droxyproline was detectable after both soaking conditions on virgin or bleached/waved hair tresses not treated with the collagen hydrolysate. It is generally known that hair (keratin) protein does not contain hydroxyproline (14). In contrast, hair tresses treated with the collagen hydrolysate and subjected to both soaking conditions had hydroxy- proline-containing peptides in each fraction (see Table II). As indicated in Table II, about twice as much hydroxyproline-containing peptides were released from virgin hair in the high-temperature soaking than from the high-salt soaking, whereas, in the bleached/waved hair, about fourfold higher levels of hydroxy- proline were present in the high-temperature soaking fractions than in the high-salt conditions. Similar results were obtained with different preparations of protein hydrol- ysate. Our observations support earlier results that bleached/waved hair binds more hydroxyproline-containing peptides than virgin hair (1,2,6). Furthermore, the total hydroxyproline released from the same weight of hair in both soakings was approxi- mately the amount observed in previous work quantitating hydroxyproline-containing peptides bound to hair (2). In separate experiments, we assayed base-hydrolyzed samples of hair tresses for residual hydroxyproline after both the high-temperature and high-salt soakings. The results demonstrate that no detectable hydroxyproline remained on the hair, implying that the soaking conditions were sufficient to remove quantitatively all of the bound hydroxy- proline-containing collagen hydrolysate (see Table II). MW CHARACTERIZATION OF PEPTIDES REMOVED FROM BLEACHED/WAVED HAIR To further characterize the substantive collagen peptides removed from hair tresses, we first chromatographed the intact solution of the collagen hydrolysate on a Sephadex G-50 column prior to incubation with the hair tress. As shown in Figure 1A, there was a wide distribution of peptides between MWs of 1,000-30,000, as indicated by fluo- rescent labeling of the peptide amino groups in select fractions. Also shown are select Table II Quantitation of Hydroxyproline in Peptide Fractions Removed From Hair Tresses Hair tress Temp. Hydroxyproline (txg) Removed peptides 0.5 M NaC1 Virgin (- protein hydrolysate) Bleached/waved (- protein hydrolysate) Virgin (+ protein hydrolysate) Bleached/waved (+ protein hydrolysate) Bleached/waved hair tress treated with protein hydrolysate, washed/removed N.D. N.D. N.D. N.D. 41.5 •g 25 •g 153.0 •g 4O •g N.D. N.D. = not detected. The hydroxyproline assay was done on the concentrated and lyophilized fractions as described in Materials and Methods. Each point represents an average of duplicates at two different concentrations and quantified relative to a standard curve for hydroxyproline. Each hair tress was approximately 3.0 grams.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)