42 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS were chromatographed on a G-50 column as shown in Figure 1C. In contrast to the high-temperature-removed peptides, a relatively high content of high-MW fluoresca- mine-reactive peptides were present at the void volume of the column (30,000 MW). The lower MW, high-salt-removed peptides were much broader in MW distribution than the high-temperature-removed peptides (see Figure lB,C). Despite the relatively low level of hydroxyproline in the high-salt-removed fraction (see Table II), the G-50 column fractions were assayed for the presence of this amino acid. Preliminary charac- terization of the hydroxyproline content of the peptide(s) present in select column frac- tions indicates that low levels of this amino acid were present at both the 30,000- MW and 1,000-MW positions (see arrows, Figure 1C). Because the high-salt fraction contained lower levels of both fluorescamine-reactive peptides and hydroxyproline, a more definitive characterization will require isolation of larger quantities of material. The results in Figure 1 demonstrate the selective binding of peptides of more defined MWs to hair tresses from a complex mixture of a cosmetic grade collagen hydrolysate. Furthermore, the qualitative differences in the MW profiles of the two different methods for removing bound peptides from hair are consistent with the idea that the final MW of the collagen hydrolysates is an important criterion for peptide binding to bleached/waved hair. It should be noted that preliminary observations suggest a similar peptide MW distri- bution for fluorescamine-reactive collagen peptides removed from virgin hair treated with protein hydrolysate (data not shown). CHARACTERIZATION OF LOWER MW HYDROXYPROLINE-CONTAINING PEPTIDES Qualitative differences in the MW distribution of the high-temperature and high-salt peptide(s) soakings were more apparent when we fractioned each sample from damaged hair on Sephadex G-15. As shown in Figure 2A, the high-temperature-removed frac- tion has a broad distribution of fiuorescamine-detectable peptides between 200-1,500 MW. In contrast, when the 0.5 M NaCl-removed fraction was chromatographed on G-15 (see Figure 2B), only high-MW peptides (1,000) were detected. When both column fractions were assayed for the presence of hydroxyproline, we observed very distinct patterns (see Figure 2A,B). The high-temperature-removed peptides contain a much higher content of 1ow-MW peptides (di- and tri-peptides) with a relatively high content of hydroxyproline. In contrast, within our detection limits, the peptides re- moved from hair tresses with high salt contain very low quantities of both 1ow-MW (500 daltons) fiuorescamine-detectable peptides, and 1ow-MW hydroxyproline-con- raining peptides. These results extend previous studies on the binding of hydroxyproline-containing pep- tides to virgin and bleached/waved hair by demonstrating the selective adsorption of peptide(s) via size (MW) and presumed charge to hair keratin. In the current study using a cosmetic grade collagen hydrolysate, it is clear that there are at least two dis- tinct populations of collagen peptides that bind to the hair: high-MW (30,000) and 1ow-MW (1,000-3,000). The majority of the hydroxyproline-containing peptides that bind to bleached/waved hair is in the lower MW range. Previous results demonstrate that adsorption of collagen hydrolysates to hair keratin increases with decreasing MW of the peptides (1). We presume that the high tempera-
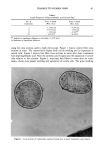
COLLAGEN PEPTIDE SUBSTANTIVITY TO HAIR 43 BO 50 10 V o Vi ,I, ,I, 2.0 o 1.5 • 0.5 • e• ß ß el e ß All ß ß ß ß 60 80 100 120 140 ß 60 B 5O 4O 3O 20 ß •0 %•' ß..• •...• ''- '' '. © A- A-- A-- A-- A-- A --• e A'•A--I'A-•- l e_ e _•_A e_ e.e Ae_ e 60 80 100 120 140 0.6 o 0.4 ,_ •_, 0.2 •øo Fraction No. Figure 2. Elution pattern from Sephadex G-15 of collagen hydrolysate peptides extracted from damaged hair. A) High-temperature or B) High-salt extraction. Monitored fluorescamine (© -- ©) and hydroxypro- line (A -- A) content of protein fractions placed on a G-15 column. Relative fluorescence and hydroxypro- line content in select fractions were monitored as described in Materials and Methods. ture swells the hair structure, allowing exit of adsorbed peptides from the hair shaft, whereas the high salt removes collagen peptides bound via ionic interactions to the hair. ACKNOWLEDGMENTS We acknowledge the work of Dave Simnick in earlier studies related to peptide sub- stantivity to hair. We thank Hank Weightman and Ron Caesar for preliminary amino acid analysis of the peptide fractions. We also thank Barbara Berk for assistance in preparation of this manuscript. REFERENCES (1) E. S. Stern and V. L. Johnsen, Studies on the molecular weight distribution of cosmetic protein hydrolysates, J. Soc. Cosmet. Chem., 28, 447-455 (1977).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)