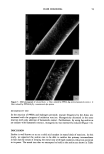
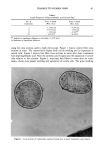
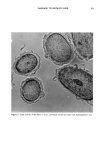
DAMAGE TO HUMAN HAIR 67 simultaneously is in agreement with the conclusion that different bonds are primarily responsible for the wet and the dry tensile properties of human hair. CONCLUSIONS Oxidation of hair fibers with diperisophthalic acid can produce extensive damage to the cuticle that can be readily observed microscopically. At the same time, no detectable changes in the tensile properties (wet or dry) are detectable. These results are consistent with the hypothesis that the tensile properties of human hair are due primarily to the cortex, with essentially no cuticle involvement, and support the two-phase model of Feughelman (4) that explains the mechanical properties of human hair in terms of its cortical components. Furthermore, these results show clearly that one cannot rely on the tensile properties alone to assess damage to human hair. This study shows that it is possible to produce extensive damage to the cuticle with no detectable changes in the tensile properties of human hair. LITERATURE CITED (1) C. R. Robbins, in "Chemical and Physical Behavior of Human Hair," 2nd ed. (Springer-Verlag,, New York, 1988), p. 226. (2) L.J. Wolfram and M. K. O. Lindemann, Some observations on the hair cuticle, J. Soc. Cosmet. Chem., 22, 839-850 (1971). (3) D. R. Rao and S. K. Chopra, The effect of the medulla on the stress-strain properties of wool fibers, J. Text. Inst., 78, 306-308 (1987). (4) M. Feughelmann, The physical properties of alpha-keratin fibers, J. Soc. Cosmet. Chem, 33, 385-407 (1982). (5) K. Allworden, Properties of wool--Detection of damaged wool by chemical means, Z. Angew. Chem. 29, 77 (1916). (6) P. Alexander, M. Fox, and R. Hudson, Reaction of oxidizing agents with wool. V. Oxidation products of the disulfide bond and the function of a sulfonamide in the peptide chain, Biochem. J., 49, 129 (1951). (7) C. R. Robbins, in "Chemical and Physical Behavior of Human Hair," 2nd ed. (Springer-Verlag, New York, 1988), pp. 231-232.
j. Soc. Cosmet. Chem., 42, 69 (January/February 1991) Letter to the Editor TO THE EDITOR: Perusal of a regression study carried out during a Canadian winter brought to mind the frequent inappropriate use of relative humidity when examining physiological phe- nomena occurring at widely different temperatures. The purpose of this note is to sug- gest that absolute humidity should be more generally used. The water content of the atmosphere can be expressed either as absolute or as relative humidity. ß Absolute humidity is the amount of water vapor in unit volume of air and is given, for example, in gm per m 3. ß Relative humidity is the ratio of the actual vapor pressure of the water in the air to its saturated vapor pressure at the same temperature. This ratio is readily measured by simple devices such as the hair hygrometer and the psycho-meter, which doubtless accounts for its frequent use. The saturation vapor pressure of air is a function of temperature. Thus at - 10øC, it takes 2.83 gm of water vapor to saturate 1 m 3 of air, while at 40øC this takes 51.27 gm of water vapor. Consequently, a sample of air containing 2.83 gm per m 3 of water vapor will have a relative humidity (RH) of 100 percent at - 10 ø, but its RH at 40øC will only be 21.5 percent. The rate of evaporation is inversely proportional to the water content or absolute hu- midity of the air. The higher the humidity, the lower the evaporation rate. When the temperature is constant, the RH is directly related to the absolute humidity. This is clearly not the case when the temperatures at which comparisons are made are not the same. The greater the temperature difference, the more important this diver- gence becomes. Since the human body, including the skin, is generally not at the same temperature as its environment, the use of relative humidity is, strictly speaking, meaningless and should be replaced by absolute humidity. Franklin J. Wright, Ph.D. 69
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)