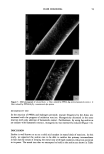
36 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS taining peptides substantive to hair. Using two different soaking methods--high-tem- perature (50øC) and high-salt (0.5 M NaCl)--we have removed hydroxyproline-con- taining peptides bound to hair. Quantitative and qualitative differences in both peptide fractions were characterized using various analytical techniques. MATERIALS AND METHODS PRETREATMENT OF HAIR TRESSES Hair tresses of virgin brown DeMeo human hair were made in 7-inch lengths weighing approximately 3 grams. After the tresses were prepared and prior to treatment, they were washed by immersion in a 1% solution of Triton X-100 solution, rinsed thor- oughly with warm tap water, and allowed to dry in a 48øC oven as described previously (6,7). Damaged hair tresses were prepared by immersing hair tresses in an alkaline solution of hydrogen peroxide at 32øC for 30 minutes. They were then washed and immersed for 20 minutes at room temperature in a 5% thioglycollate solution, water rinsed, and neutralized with a solution of 3% hydrogen peroxide by soaking for 12 minutes. Each hair tress was rinsed and allowed to dry. Both the virgin (natural) and bleached/waved (damaged) hair were washed with a 1% solution of Triton X-100 and dried as above before each treatment with protein. ABSORPTION AND REMOVAL OF PROTEIN/PEPTIDES FROM HAIR TRESSES Each hair tress (approximately 3 g) was soaked in a 5% (wt/vol) solution of collagen protein hydrolysate (LEXEIN © X-250, a registered trademark for an aqueous solution of hydrolyzed collagen protein) for 10 minutes at 25øC. To remove nonspecifically adsorbed protein, the hair was rinsed with warm running tap water for 1 minute and squeezed to remove excess water. Removal of bound collagen peptides from the hair tresses was accomplished by sequential steps in the following solutions: 1) each swatch (3 g) was placed in 30 ml of deionized water and then put in a 50øC water bath for 45 min 2) the hair swatches were subsequently soaked overnight at 25øC in 30 ml ofa 0.5 M NaCI solution to remove any remaining peptides. Both virgin and bleached/waved hair tresses were subjected to the high-temperature and 0.5 M NaCI soakings in tripli- cate to compare variations from one tress to another. No experiments were done re- versing the sequence of the soaking conditions. CONCENTRATION OF REMOVED PEPTIDES The separate fractions of high-temperature- and 0.5 M NaCl-removed peptides were concentrated by lyophilization. The dried powders were resuspended in deionized water to small volumes (1- 2 ml) and stored in a freezer at - 4øC prior to further characteriza- tion and analysis. GEL FILTRATION The molecular weight distribution of peptides removed from the hair tresses was deter- mined by standard gel filtration procedures on Sephadex G-50 and G-15 sizing columns. The columns were equilibrated in 0.15 M NaCI and 0.1% SDS, and fractions
COLLAGEN PEPTIDE SUBSTANTIVITY TO HAIR 37 of 1 ml were collected. The MW standards for the G-50 column were as follows: ovalbumin (43K), cytochrome C (12K), bradykinin (1,250) and glycyl-glycyl-glycine (171) and for the G-15 column: insulin B-chain (3,500), bradykinin (1,250), glycyl- glycyl-glycine (171). The void volumes and included volumes were determined with blue dextran, and phenol red or tyrosine, respectively, under identical column condi- tions. The presence of peptides (amino groups) at specific MW ranges was determined by fluorescamine labeling of the columns' fractions (1 ml) after chromatography of the peptides. Similarly, the MW distribution of peptides derived from collagen was deter- mined by hydrolyzing select fractions (1 ml) and quantitating the hydroxyproline con- tent (9). Approximately 1,000-1,500 relative fluorescent (RF) units of the high-tem- perature soaking and the 0.5 M NaCI soakings were chromatographed on both the G-50 and G-15 Sephadex columns. FLUORESCENT DERIVATIZATION OF PEPTIDES The presence of primary amine groups in the high-temperature and high-salt soaked fractions from the lyophilized and gel filtration columns was determined by fluoresca- mine reaction with samples accoMing to previous procedures (10-13). The relative fluorescence was measured on a Gilford Fluoro IV fluorometer. The excitation and emission wavelengths were set at 390 nm and 480 nm, respectively. A freshly prepared solution of fluorescamine was used for all amino group derivatizations. HYDROXYPROLINE ASSAY Hydroxyproline assays of individual column fractions or of the substantive peptides removed from the hair tresses were determined by base hydrolysis of the collagen pep- tides followed by hydroxyproline analysis as described previously (8,9). Select column fractions were lyophilized to concentrate the material prior to hydrolysis and subsequent hydroxyproline analysis. The relative hydroxyproline content of the various peptide fractions was determined relative to a standard curve for hydroxyproline. Absorbance measurements for the quantitation of hydroxyproline levels were done on a Gilford Spectrophotometer Model 260. To determine the background absorbance (hair tress never treated with the protein hydrolysate) and demonstrate that no hydroxyproline remained on hair tresses soaked in high temperature and 0.5 M NaCI, samples of hair tresses (10 mg) before and after the protein hydrolysate were hydrolyzed and assayed for hydroxyproline. MATERIALS Fluorescamine and all other reagents were obtained from Sigma Chemical. The lyophi- lizer was a Virtis Model 112920 Bench Top 3 from American Scientific Products. Sephadex G-50 and G-15 were purchased from Pharmacia. RESULTS AND CONCLUSIONS REMOVAL OF PEPTIDES FROM HAIR Virgin (natural) and bleached/waved (damaged) hair tresses were incubated with a cos-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)