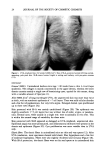
TRICLOSAN ASSAY 41 A [] 4 I• 4 I• rain Figure 3. HPLC trace of a deodorant soap preparation purified (A) by SFE (0.103 g) or (B) by the method reported in the literature (6) (0. 120 g). Conditions and peak identification are as in Figure 2 2 = triclocarban. CONCLUSIONS In conclusion, the first SFE method has been developed for the rapid and selective isolation of triclosan from deodorant sticks and soaps. The proposed procedure provides a higher degree of sample purification than classical liquid extraction (6). Since SFE allows the automation of the entire extraction process, improved precision and faster method development are achieved, in comparison with conventional manual sample preparation. Moreover, the drastic reduction of hazardous solvents required represents another advantage of SFE. Because of minimal sample handling, ease of operation, good accuracy, and reproducibility, the SFE procedure is suitable for routine quality control analyses of triclosan in deodorants, particularly to verify the conformance of the com- mercial products to the current legislation. ACKNOWLEDGMENTS The authors thank Hewlett-Packard (Italy) for the loan of the HP 7680A instrument. Financial support from the Consiglio Nazionale delle Ricerche, Piano Finalizzato Chim-
42 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ica Fine e Secondaria, is gratefully acknowledged. The authors wish to thank G. Bonora (Physics Department, Ferrara University) for his skilled technical assistance. REFERENCES (1) A. R. Cox, Efficacy of the antimicrobial agent triclosan in topical deodorant products: Recent devel- opments in vivo, J. Soc. Cosmet. Chem., 38, 223-231 (1987). (2) C. Fox, Antiperspirants and deodorants review and update, Cosmet. Toiletr., 100, 27-41 (1985). (3) H. Bremer and W. Klein, "Deodorants," in Cosmetics and Toiletries: Development, Production and Use, W. Urnbach, Ed. (Ellis Horwood, Chichester, 1991), pp. 115-121. (4) J. N. Labows, K. J. McGinley, and A.M. Kligman, Perspectives on axillary odor, J. Soc. Cosmet. Chem., 34, 193-202 (1982). (5) C. Fearnely and A. R. Cox, A new microbiological approach to the assessment of underarm deodor- ants, Int. J. Cosmet. Sci., 5, 97-109 (1983). (6) R. G. Achari and D. Chin, HPLC analysis of some bacteriostats in deodorant sticks and soaps, J. Soc. Cosmet. Chem., 32, 163-173 (1981). (7) European Economic Community Council Directive 76/768/EEC, Appendix VI (1976). (8) E. Jungermann and E. Beck, Determination of germicides in soaps and detergents,J. Amer. Oil Chem. Soc., 38, 513-518 (1961). (9) M. B. Graber, I. I. Domsky, and iV[. E. Ginn, TLC method for the identification of germicides in personal care products, J. Amer. Oil Chem. Soc., 46, 529 (1969). (10) L. Gagliardi, G. Cavazzutti, L. Turchetto, F. Manna, and D. Tonelli, Determination of preservatives in cosmetic products by reversed-phase high-performance liquid chromatography. IV., J. Chromatogr., 508, 252-258 (1990). (11) L. Gagliardi, A. Amato, A. Basili, G. Cavazzutti, E. Gattavecchia, and D. Tonelli, Determination of preservatives in cosmetic products by reversed-phase high-performance liquid chromatography. II., J. Chromatogr., 325, 353-358 (1985). (12) S. B. Hawthorne, Analytical-scale supercritical fluid extraction, Anal. Chem., 62, 633A-642A (1990). (13) J. C. Giddings, M. N. Myers, L. McLaren, and R. A. Keller, High pressure gas chromatography of nonvolatile species, Science, 162, 67-73 (1968). (14) K. D. Bartie, "Theory and Principles of Supercritical Fluid Chromatography," in Supercritical Fluid Chromatography, R. M. Smith, Ed. (Royal Society of Chemistry, Cambridge, 1988), pp. 2-4. (15) S. Scalia and D. E. Games, Determination of parabens in cosmetic products by supercritical fluid extraction and high-performance liquid chromatography, Analyst, 117, 839-841 (1992).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)