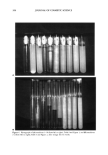
j. Cosmet. Sci., 50, 203-219 (July/August 1999) Stability factors and vapor pressures in a model fragrance emulsion system STIG E. FRIBERG and ZHIQIANG ZHANG, Department of Chemistry, Clarkson University, Potsdam, NY 13699-5810 LI GANZUO, Institute of Colloid and Interface Chemistry, Shandong University, Jinan, Shandong 250100, China and PATRICIA A. AIKENS, Uniq6wza, 3411 Silverside Road, Wilmington, DE 19850-5391. Accepted for publication June 30, 1999. Synopsis Emulsions were prepared ofphenethyl alcohol using laureth 4, a commercial tetraethyleneglycol alkyl ether, as the stabilizer. The results showed the stability to depend critically on the ratio of surfactant to phenethyl alcohol in the sequence low-high-low-high stability. The explanation was found in the variation in density of the oil phase and in the phase changes taking place at high surfactant/alcohol ratios. With increased ratios the emulsion changed from a two-phase emulsion, oil-in-water (O/W), to a three-phase one (oil + liquid crystal)-in-water ([O + LC]/W), to a two-phase one (liquid crystal-in-water [LC/W]). The three-phase emulsions with a ratio of lameliar liquid crystal to oil phase (LLC/O) -- 1 showed excellent stability. INTRODUCTION Fragrance combinations are an essential part of personal care formulations, the fragrance perception being the decisive factor in consumer acceptance of a product. Their formu- lations are a highly sophisticated science-art (1,2), with a tradition reaching back thou- sands of years into Middle Eastern cultures, with names like Arabia (Land of Fragrance) and A1 Khimija (Alchemy) remaining as landmarks in a long development of a highly refined art (3,4). In modern times, with fragrances becoming a part of all strata of society, an inroad of science took place in the world of fragrance formulation, with highly sensitive analytical methods (5) such as the gas chromatography-mass spectrometry combination enabling the use of synthetic compounds to mimic natural fragrances (6). Another change has been caused by the regulatory agencies, whose restriction on the use of volatile organic solvents (VOC) has limited the use of alcohol in fragrance formula- tions. Most fragrance materials are strongly hydrophobic in nature and, hence, are not soluble in water, causing a problem in formulation efforts. One immediate solution is to 203
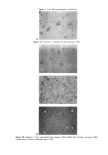
204 JOURNAL OF COSMETIC SCIENCE Table I Composition and Weight Fraction of Phases for Emulsions in Figures 4A,B Composition Weight Fraction of Phases PEA Laureth 4 H20 Emulsion (wt %) (wt %) (wt %) Aqueous Microemulsion LLC 1 5.0 0 95 0.962 0.038 0 2 4.2 0.8 95 0.961 0.039 0 3 3.4 1.6 95 0.959 0.041 0 4 2.6 2.4 95 0.933 0.067 0 5 2.25 2.75 95 0.932 0.049 0.019 6 1.8 3.2 95 0.930 0.026 0.044 7 1.45 3.55 95 0.928 0 0.072 8 1.0 4.0 95 0.920 0 0.080 9 0.5 4.5 95 0.913 0 0.087 10 0 5.0 95 0.90 0 0.10 a 2.7 2.3 95 0.953 0.047 0 b 2.55 2.45 95 0.933 0.067 0 c 2.3 2.7 95 0.932 0.052 0.016 d 2.15 2.85 95 0.932 0.046 0.022 e 2.05 2.95 95 0.931 0.040 0.029 f 2.0 3.0 95 0.931 0.035 0.034 g 1.8 3.2 95 0.930 0.026 0.044 h 1.7 3.3 95 0.930 0.018 0.052 i 1.42 3.58 95 0.927 0 0.073 j 0.8 4.2 95 0.922 0 0.078 solubilize the fragrance compounds into aqueous surfactant association structures, such as micelies (7-10), microemulsions (11,12)and liposomes (13). These different approaches have led to an emerging literature on phase diagrams of systems of water, fragrance compounds, and surfactants (14-17). The colloidal structures in these phases have been analyzed for their influence on the vapor pressure of single and combined fragrance compounds and the relation to vapor pressure during evaporation (17,18). These results form a beginning in the examination of the behavior of fragrance compounds solubilized into surfactant association structures. The fact that simple systems may actually show a constant vapor pressure during free evaporation (18) was considered sufficiently promising that an investigation into the properties of different forms of dispersed systems was considered proper and useful. As a first example we have chosen a single fragrance compound, phenethyl alcohol, because of its importance in the fragrance industry and because it has recently been investigated extensively (19). EXPERIMENTAL MATERIALS Laureth 4 (Brij©30) (Uniqema, Wilmington, DE) and phenethyl alcohol (PEA) 99% (Aldrich Chemical Co., Milwaukee, WI) were used as received. Water was deionized and doubly distilled.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)