MATRIX COMPOUNDS AND OXIDATIVE HAIR DYES 241 affect either the retention times of the dyes or their RSD, nor the mean values of the corrected peak area. Nevertheless, it is important to note that the repeatability of the measurements for one component (2-n-l,4-pd) seems to be strongly affected by the presence of BHT, with the RSD increasing from 6.4% to 21%. BHT itself has a high RSD for its peak corrected area. Regarding these results, it appears, quite clearly, that although the matrix compound EOP does not interfere with the dye intermediates or with the column in a way that would affect the chromatographic behavior of the dye intermediates, it would be of advantage to remove BHT and DMDM from the sample solution before proceeding with the separation in order to obtain an accurate separation of the dye intermediates. OPTIMIZATION OF THE LIQUID-LIQUID EXTRACTION METHOD Since several matrix compounds are present in a real formulation, it is of immense interest to determine the effect of the isolation method on each of them. The optimi- zation of the extraction protocol leads to a one-step (LDA, TDS, NNO, ORA), two-step (OA, BHT, DMDM, NaAsc), or three-step (CC, DC) extraction by n-heptane, while EOP, NOL, LS, SS, TEA, PQ, MP, and PVP are not extracted. Two different approaches were considered for testing the extraction method. BHT and EOP were submitted to extraction in a mixture containing the dye intermediates as well, whereas in a second approach, the extraction was performed on single solutions of matrix compounds. Extraction of sample solutions containing a matrix compound and three dye intermediates. The extraction protocol has to be efficient to extract the matrix compounds, of course, but the target dyes should not be extracted. A one- to three-step extraction protocol was thus applied to sample solutions containing three selected dye intermediates (4-ap, res, and 2-n-l,4-pd), the antioxidant NaAsc, and a major matrix product (BHT or EOP). The aqueous phases obtained were submitted to HPLC, and the extraction yield was calcu- lated according to the following equation: ( corrected area after extraction ) yield (%) = 100 - k, correcte-------• re--•efor-•' extractio---•-• X 100 (1) Table V gives the extraction yield for each component in the solution, and the successive chromatograms obtained for the solution containing BHT before (a) and after (b) the extraction procedure are shown in Figure 3. First of all, it is clearly seen that the extraction procedure affects neither the chromatographic characteristics of the dye in- Table V Statistical Extraction Yield of the Components of a Dye-EOP and a Dye-BHT Mixture Compound One-step extraction yield (%) Two-step extraction yield (%) 4-ap 0 0 Resorcinol 0 0 2-n- 1,4-pd 0 0 NaAsc 19 50 EOP -- 0 BHT 89 100 (n.p.) n.p.: no peak.
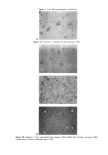
242 JOURNAL OF COSMETIC SCIENCE 550, 250__ -- -60 4 250-- Retention time (rain) 550 4 I -- 2 , I -60 0.0 15.0 30.0 49.0 Retention time (rain) Figure 3. Successive chromatograms of a dye intermediate mixture with BHT added to the sample before and after extraction. a: Before extraction. b: After a two-step extraction. Peaks: (1) NaAsc (intrinsic matrix, antioxidant) (2) 4-ap (3) res (4) 2-n-l,4-pd and (5) BHT (matrix, antioxidant). Peaks 2, 3, and 4 are unchanged between steps a and b. (See text for experimental data and abbreviations.) termediates (retention times, corrected peak areas of peaks 2, 3, and 4) nor the repeat- ability of the analysis. Then, after a two-step extraction by n-heptane, 50% of the antioxidant NaAsc is extracted while 100% of BHT (peak 5) is extracted from the sample. However, it appears that EOP is not at all extracted by n-heptane. Additional experimental work has shown that EOP was 100% extractable by other tested organic solvents (trichloromethane, dichloromethane, or diethylic ether, for instance) but that they also lead to the partial extraction of the phenolic dyes (data not reported). This extraction procedure by n-heptane is therefore very effective for the separation of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)