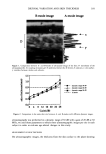
j. Cosmet. Sci., 52, 369-375 (November/December 2001) A modified cup scrub method for assessing the antibacterial substantivity of personal cleansing products WARD L. BILLHIMER, CINDY A. BERGE, JAN S. ENGLEHART, GAYLE Y. RAINS, and BRUCE H. KESWICK, The Procter O Gamble Company, Cincinnati, OH 45241. Accepted for publication August 15, 2001. Synopsis An improved in vivo method for evaluating the antibacterial substantivity or residual effectiveness of bar soaps and other personal cleansing products is presented. The effectiveness of an antibacterial bar soap containing 1.5 % 3, 4, 4'-trichlorocarbanilide (TCC) versus its soap vehicle was evaluated under simulated conditions considered optimal for bacterial growth, proliferation, and possible infection. A washout period to clear the skin of any antimicrobial agents previously used was followed by a treatment period in which the subjects washed one of their forearms with the antibacterial soap and the other forearm with the soap vehicle. Either immediately or 24 hours following the final wash, three test sites on both forearms were inoculated with S. aureus and occluded with Hill Top Chamber © patches. At intervals of 30 minutes, two hours and five hours, the patches were removed. The bacteria on the skin were harvested using the Williamson-Kligman scrub technique (1,2) to determine the number of surviving CFUs at each time period. The method successfully demonstrated that sufficient TCC had remained on the skin for 24 hours after the final wash to effectively inhibit the growth ofS. aureus on the skin for as long as five hours after inoculation. INTRODUCTION Skin infections due to Staphylococcus aureus such as folliculitis, furuncles, carbuncles, impetigo, and wound infections, are recognized as a common health problem (3,4). The regular use of an antibacterial soap for personal cleansing has the potential to control the growth of S. aureus on the skin and to aid in the prevention of these diseases. The surfactant base removes bacteria from the skin while depositing an active ingredient that can help to control the regrowth of remaining organisms and help to prevent the colonization of other transient, potentially pathogenic organisms. Demonstrating the disease prevention potential of an antibacterial soap under consumer conditions requires significantly large numbers of subjects and extensive amounts of time, and it is often financially prohibitive. Instead, it is useful to demonstrate the benefits of washing with antibacterial soaps by using controlled clinical methods designed to demonstrate the differences between plain soap and water (placebo) and the product containing an antibacterial agent. 369
370 JOURNAL OF COSMETIC SCIENCE Various clinical methods for evaluating the efficacy of antibacterial soap products have been reported in the literature (1,5-7). These methods typically measure the ability of a product to reduce the resident flora through a continuous action or evaluate the ability of a product to reduce the level of marker organisms on artificially contaminated hands. These methods, however, do not measure the ability of an antibacterial agent to remain on the skin for a prolonged period and continue suppressing growth of acquired skin pathogens in situ. This antibacterial substantivity or residual effectiveness is an impor- tant characteristic to measure in evaluating the overall performance of an antibacterial product. Aly and Maibach (8) reported the development of a method to evaluate the residual effectiveness of a single application of topical antibacterial products against various skin pathogens. This method incorporated the occlusion of treated skin sites inoculated with bacteria and the enumeration of the bacteria remaining after five hours of occlusion via the cup scrub technique. Residual antibacterial effectiveness was determined by com- paring the differences in growth and survival of the test organism on sites treated with either the antimicrobial agent or a vehicle. Finkey et al. (9) and Scala et al. (10) used this method to evaluate antimicrobial soaps following repeated application. These studies did not, however, establish how quickly the antibacterial effect occurs or how long it lasts. Our objective was to determine the rate and duration of the antibacterial action of antibacterial soaps under simulated use conditions. We modified the previously reported residual effectiveness test procedure (9,10) by inoculating the sites either immediately or 24 hours after the final wash and harvesting the surviving organisms after various intervals of occlusion. MATERIALS AND METHODS Two studies illustrate the method, one study examining the immediate antibacterial efficacy of the bar soap containing 1.5% TCC relative to the vehicle bar, while the second study examines the prolonged antibacterial efficacy of the same two bars. STUDY POPULATION Twenty (20) subjects, male and female, ages 18 to 65 years, were enrolled in each study. These subjects were screened for normal skin on the forearms. In order to clear the skin of any residual antimicrobials previously used, subjects were instructed to use only the non-medicated personal cleansing bar soap provided in place of their regular personal cleansing product during a 7-14-day washout period. They were also instructed to refrain from using any antibacterial soaps, medicated lotions and creams, and dandruff shampoos until the study was completed. All subjects provided written, informed consent. TEST MATERIALS Two bar soaps were examined in each study. The antibacterial soap contained 1.5% 3, 4, 4'-trichlorocarbanilide (TCC). The other soap was the vehicle soap bar containing no antimicrobial agents.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)