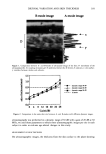
2001 ANNUAL SCIENTIFIC SEMINAR 411 upon application of two lotions. The nano-dispersion lotion showed uniformly spread intact particles (Fig. 2A) where as the regular lotion showed uneven spreading with localized blobs (Fig. 2B). Figure 2 suggests that even after most of the water from lotion evaporates upon application to skin, particles in nano- dispersion lotion remain intact and form a uniform film on the skin surface where as uneven spreading with undefined particles results from application of conventional lotion. This indicates that particles in conventional lotion are susceptible to change in environment. '•. • •' • •'.• • • • - A B Figure 2: Environmental Scanning Electron Micrographs of the in-vitro skin surface applied with sunscreen lotions. A: Lotion with nano-dispersion, B: conventional sunscreen lotion. It is reported that sunscreens formulated with conventional emulsifying agents loose considerable amount of actives (36% loss of parsol 1789) after exposure to UV radiation (Deflandre and Lang, 1988). Figure 3 shows that in nano-dispersion lotion, 75-80% of the sunscreen actives remained intact after 2hrs of exposure to bright natural sunlight while only 60-65% of the actives remained intact in the same period from a conventional lotion. This suggests that a direct relationship exist between UV protecting ability of the sunscreen agents and the size, homogeneity, and stability of the particles after application on skin. Our studies suggest that if a sunscreen formulation spreads on skin to form a uniform film of intact, homogeneous nano-particles it is likely to protect the skin from UV radiation for a considerable time period •roviding a high SPF. • •ø•---•c •a• Figugre 3: Recovery of the z m • exposure to natural bright •0 sunlight. Curvel: From nano- 0 •0 m • ,•0 • dispersion lotion, Curve 2: o 3o •0 •o • •s0 •,,•.•n From conventional lotion CONCLUSIONS ß Nano-dispersion produced by proprietary technology showed uniform particle size in the range of 300- 600 nm ß Our studies showed that lotion made from nano-dispersion spread uniformly on skin compared to the lotion of the same ingredients made in a conventional manner ß Our studies indicated that the integrity of the sunscreen actives is strongly dependent on the formulation. Actives from nano-dispersion lotion were significantly stable compared to the same actives from lotion made in a conventional manner ß Our studies suggest that formation of a uniform film with intact nano-particles is the key for achieving nearly two-fold SPF improvement over conventional lotion REFERENCES Cole, C., (2001) "Sunscreen protection in the ultraviolet A region: how to measure the effectiveness" PhotodermatoL Photoimmunol. Pho toreed. 17:2-10 Deftandre, A, and Lang, G. (1988) "Photostability assessment of sunscreens. Bcnzylidcnc camphor and dibcnzoylmcthanc derivatives" Intl. J. Cosmet. Sci. 10, 53-62 Jiang, R., Hay&n, C.G., Prankerd, R.J., Roberts, M.S., and Benson, H.A. (1996) "High-performance liquid chromatography assay for conunon sunscreening agents in cosmetic products, bovine serum albumin solution and human plasma" J. Chromatogr. B Biotaed. AppL 682, 137-145. * This paper is being presented by Jennifer Corwin on behalf of The Collaborative Group.
412 JOURNAL OF COSMETIC SCIENCE SUNSCREEN PHOTOSTABILITY AND UVA PROTECTION Joseph W. Stanfield Suncare Research Laboratories, LLC, 740 East Brookhaven Circle, Memphis, TN According to the FDA Sunscreen Monograph, the sun protection factor (SPF) of a sunscreen is measured in vivo on the skin of human volunteers using a solar simulator. The solar simulator UV spectrum resembles that of sunlight at high sun angles, which occur in mid-summer. At lower sun angles the actual SPF of a sunscreen in sunlight is lower than its labeled value, unless the product provides "broad spectrum" protection, that is, a substantial level of UVA protection. In addition, if the sunscreen is not photostable, its SPF and UVA protection may diminish rapidly in sunlight. This presentation describes an m vitro method for assessing sunscreen UVA protection and photostability. Since many sunscreen formulas are not photostable, any method for assessing UVA protection must take into account the photostability of the product, to be valid. In vitro measurements of sunscreen protection simulate the in vivo SPF test by using a measured amount of sunscreen applied to an artificially prepared substrate, instead of living skin. The SPF of the substrate and the SPF of the sunscreen are determined by measuring the UV transmission of the substrate alone and the substrate containing the sunscreen, as shown below: o 'Is Ess Sunscreen Substrate I0 is the effective UV irradiance applied to the surface of the substrate alone and Is is the effective UV irradiance transmitted by the substrate alone. Io and Is are obtained by measuring spectral irradiance values from 290 to 400 nm, multiplying by the CIE erythemal effectiveness spectrum and integrating over wavelength to calculate irradiance. The SPF of the substrate, SPFs = IdIs, and is assumed to be constant. E0 is the measured effective UV dose from 290 to 400 nm applied to the surface of the sunscreen on the substrate Ess is the measured effective UV dose from 290 to 400 nm
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)