400 JOURNAL OF COSMETIC SCIENCE of the Council of the European Communities (27 July 1976) was published (5). Stannous fluoride was slightly mutagenic in the Ames test. Today, over 90% of the dentifrices sold in Taiwan contain fluoride from one of two major sources (sodium fluoride and MFP). There are reports that a mixture of sodium fluoride and MFP is superior in efficacy to MFP alone in a dentifrice base (4). Dentifrices with mixed fluoride systems been marked. Quantitative determination of fluoride and MFP are important for quality control and stability evaluation of these products. Gas chromatographic (6-8), fluoride-ion electrode (6,8,9) high-performance liquid chro- matographic (10), and ion chromatographic (11,12) techniques have been used to de- termine fluoride derivatives in toothpastes. Gas chromatographic methods involve the chromatographic analysis of trimethyl fluorosilane resulting from the reaction of tri- methyl chlorosilane with fluoride ions in the toothpaste. A fluoride-ion electrode tech- nique was used only for the determination of soluble fluoride derivatives. However, MFP is soluble in water to the extent of 42% saturation and is slowly hydrolyzed in the presence of hydrochloric acid. These methods are laborious and time-consuming. The use of high-performance liquid chromatography required a postcolumn detection system in order to analyze orthophosphate, diphosphate, triphosphate, and cyclotriphosphate in MFP samples (10). Ion chromatographic methods for direct determination of MFP in toothpaste have been reported (11,12). However, oral hygiene formulations commonly contain abrasives (phosphate), an antibacterial agent (cetylpyridium chloride), astringent salts (zinc chloride), and a surfactant (sodium lauryl sulfate). These excipient anion ions were found not to be ideal for resolving fluoride and phosphate at pH 5.3-5.7. This present paper describes the application of various pH to separate rapidly and efficiently the peaks of interest. The results were compared with those obtained with the fluoride- ion electrode method. EXPERIMENTAL ION CHROMATOGRAPHY The liquid chromatograph was the Shimadzu LC-10 AD chromatography module con- taining the pump and conductivity detector (Shimadzu CDD-6A). The analytical col- umn used a Shim-Pack IC-A1 (4.6 mm ID x 10 cm) polymethacrylate-based anion exchanger (10 l•m). Chromatographic data were collected and analyzed with a Chroma- topac C-R6A. The following chromatographic conditions were used: eluent flow rate, 1.5 ml min -•' conductivity temperature, 40øC sensitivity, 1.0 l•s cm -•' injection volume, 50 lal recorder chart speed, 5 mm/min -• All reagents used were analytical grade. The MFP was obtained from Aldich Chemical Corporation and had an assay value of 95%. The pH of the eluent, containing 0.94 m mol 1 -• sodium carbonate, was adjusted to 9.85 and 11.00 with 0.31 m mol 1 -• sodium hydrogen carbonate and 1.0 mol 1-1 sodium hydroxide, respectively. The standard fluoride (1000 mg 1-•), chloride (1000 mg l-i), sulfate (1000 mg 1-•), and MFP (0.1%) solutions were prepared by dissolving the appropriate amounts of sodium salt in double deionized water. A 500-mg amount of toothpastes or mouthwashes was accurately weighed into a beaker, and 20 ml of deionized water was added and stirred until the sample was fully dispersed.
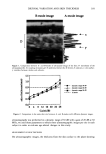
FLUORIDES IN ORAL HYGIENE PRODUCTS 401 After centrifugation, the supernatant liquid was transferred into a 50-ml volumetric flask and made up to volume with double aleionized water. An aliquot of the dispersion was filtered through a 0.2-pro (Gelman Sciences, Super Acrodisc) filter for analysis. FLUORIDE-ION ELECTRODE TECHNIQUE An amount of 2.0 g of the sample toothpastes or mouthwashes was weighed into a 50-ml beaker, and 6 ml of deionzied water was added and stirred until the sample was fully dispersed. After centrifugation, the supernatant liquid, after the treatment with hydro- chloric acid to hydrolyze the FPO3 2- ions, was made up to volume with deionized water. A 1.0-ml aliquot of the acid liquid (soluble fluoride) was neutralized with 4 tool 1-1 sodium hydroxide. The neutralized solution of sample was mixed with sodium acetate buffer (pH 4.82) containing 0.1% CDTA [(+)trans-l,2-diaminocyclohexane- N,N,N',N'-tetraacetic acid monohydrate] or TISAB I (0.50 tool 1 -• sodium chloride, 0.25 mol 1-1 trisodium citrate, and 0.50 tool 1-1 acetic acid) or TISAB II (1.83 mol 1-1 sodium acetate and 0.0052 tool 1 -• citric acid). The soluble fluoride concentration was determined with an Orion Research model 96-09 fluoride-ion electrode and an Orion model 420 mV meter. Calibration graphs were constructed using a standard solution of sodium fluoride. RESULTS AND DISCUSSION OPTIMIZATION OF THE MOBILE PHASE Dentifrices or mouthwashes containing anions include the fluoride, MFP, phosphate, and chloride in many commercial ingredients, and sulfate as an impurity in some anionic surfactants. The separation mixture of the anions of the short run times is difficult. Separation and quantification of fluoride and monofluorophosphate (FPO3 2-) was inter- ference-free in the wide range of separation conditions selected. At the elevated mobile phase pH value, an HPO42- peak was shifted away from the FPO32- peak. Carbonate buffer with pH 11.00 gave good separations of F- and FPO32-, but the overall analysis time was longer than with pH 9.85. The carbonate buffer (pH 9.85) contained a mixture of 0.94 m mole Na2CO 3 and 0.31 m mole NaHCO 3. The CO32- concentration helped achieve a larger resolution between FPO32- and SO42-. Chloride was also eluted in a chromatographic region free of interference from other ion peaks. The retention times were 2.683, 5.448, 8.473, and 11.393 rain for F-, CI-, FPO3 2-, and SO42-, respectively. Optimization of CO32- concentration and the eluent pH for maximum resolution at minimum total run time resulted in the eluent described in the Experimental section. A chromatogram of a standard solution mixture produced with this eluent is shown in Figure 1. Under these conditions separation was fast, reproducible, and free from inter- ference from other components of the sample. REPRODUCIBILITY AND ACCURACY Determination of the concentratin of the various chemotherapeutic agents was accom- plished by means of a calibration graph. The calibration graphs were linear for two chemotherapeutic agents over the range of concentration used (5.0-40.0 mg l-P). The correlation coefficients were within the range of 0.9990-0.9998. Recovery tests were
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)