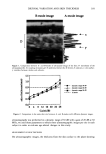
402 JOURNAL OF COSMETIC SCIENCE I ) i ..j 0 4 8 12 16 Time( min ) Figure 1. Chromatogram of standard solution using sodium carbonate buffer (pH 9.85). Mobile phase. Peaks: a = fluoride (25 mg/1) b = monofluorophosphate (30 mg/1). carried out on oral hygiene products to evaluate the reproducibility and accuracy of the proposed ion chromatographic (IC) method. Four toothpastes and mouthwashes were spiked with the amounts reported in Table I and subjected to the whole procedure. As
FLUORIDES IN ORAL HYGIENE PRODUCTS 403 Table 1. Recovery and Reproducibility in the Analysis of Toothpastes and Mouthwashes by Ion Chromatography (5 determinations for each aspect of procedure) Sodium fluoride Sodium monofluorophosphate Added Found Recovery Added Found Recovery (mg 1 -•) (mg 1 •) (%) (mg 1 t) (mg 1 -•) (%) Toothpaste A 2.00 2.07 103.5 (1.4) a 5.00 5.10 102.0 (2.3) Toothpaste B -- -- -- 5.00 5.07 101.5 (5.0) Mouthwashes A 5.00 4.87 97.3 (3.3) -- -- Mouthwashes B 4.00 3.99 99.8 (1.3) -- -- Standard deviation. shown in Table I, excellent recoveries and precision were observed (recoveries ranging from 97.3% to 103.5%). APPLICATION TO ORAL HYGIENE PRODUCTS The proposed IC method was applied to the determination of chemotherapeutic agents in oral hygiene products (toothpastes and mouthwashes). A representative chromato- gram of a commercial toothpaste is shown in Figure 2. Analytical results are given in 0 4 8 12 Time( min ) Figure 2. Chromatogram of typical toothpaste using sodium carbonate buffer (pH 9.85). Mobile phase. Peaks: a = fluoride b = rnonofluorophosphate.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)