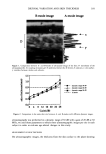
DIURNAL VARIATION AND SKIN THICKNESS 397 Concerning the effects of the time of measurement on age-related changes in skin thickness (diurnal variations), such changes were observed in the forehead, the corners of the eye, and the forearms in the morning but not in the afternoon. However, the cheeks showed an age-related increase in skin thickness in both the morning and the afternoon. Therefore, cheek skin might differ from other areas in structure and might be less affected by derreal fluid content than other facial sites. In addition, the effects of diurnal variations on age-related changes in skin thickness observed in this study may be due to an age-related increase in the degree of transfer of dermal fluid. Thus, diurnal variations are an important factor, especially when age-related changes in the skin thickness of the face are evaluated. The skin thickness of the face appears to be more markedly affected than other areas by dermal fluid, and the measurement obtained in the morning may reflect age-related changes more markedly. In the forearm, skin thickness showed an age-related decrease in the morning but no age-related change in the afternoon. In females with a mean age of 30 years who took part in this study, diurnal variations in skin thickness and the echogenicity of ultraso- nographic images were similar between the forearm and the face, showing a decrease in skin thickness and an increase in echogenicity in the afternoon (12). Skin elasticity in the forearm increased in the afternoon, but the increase was not so marked as that observed in the face (12). Other investigators have reported an increase in echogenicity in the forearm 12 hours after rising in the morning, both in young and in aged groups (14). It seems likely that in forearm skin derreal fluid might have slighter effects on age- related changes. In conclusion, when measuring skin thickness, an appropriate time for taking measurements should be selected with consideration of diurnal movements of dermal fluid. REFERENCES (1) H. Alexander and D. I. Miller, Determining skin thickness with pulsed ultrasound,J. Invest. Dermatol., 72, 17-19 (1979). (2) J. D. Rigal, C. Escoffier, B. Querleux, P. Agache, andJ. L. Leveque, Assessment of aging of the human skin by in vivo ultrasonic imaging. J. Invest. Dermatol., 93, 621-625 (1989). (3) S. Seidenari, A. Pagnori, A.D. Nardo, and A. Giannetti, Echographic evaluation with image analysis of normal skin: Variation according to age and sex, Skin Pharmacol., 7, 201-209 (1994). (4) B. D. Fornage andJ. L. Deshayes, Ultrasound of normal skin,J. Clin. Ultrasound, 14, 619-622 (1986). (5) L. O. Olsen and J. Setup, High-frequency ultrasound scan for non-invasive cross-sectional imaging of psoriasis, Acta Derm. VenereoL, 73, 185-187 (1993). (6) M. C. Branchet, S. Boisnic, C. Frances, and A.M. Robert, Skin thickness changes in normal aging skin, Gerontology, 36, 28-35 (1990). (7) C. Y. Tan, B. Starham, R. Marks, and P. A. Payne, Skin thickness measurement by pulsed ultrasound: Its reproducibility, validation and variability, Br. J. Dermatol., 106, 657-667 (1982). (8) S. Shuster, M. M. Black, and E. McVitie, The influence of age and sex on skin thickness, skin collagen and density, Br. J. Dermatol., 93, 639-643 (1975). (9) Y. Takema, Y. Yorimoto, M. Kawai, and G. Imokawa, Age-related changes in the elastic properties and thickness of human facial skin, Br. J. Dermatol., 131,641-648 (1994). (10) M. Gniadecka and G. B. E. Jemec, Quantitative evaluation of chronological aging and photoaging in vivo: Studies on skin echogenicity and thickness, Br. J. Dermatol., 139, 815-821 (1998). (11) G. Pellacani and S. Seidenari, Variation in facial skin thickness and echogenicity with site and age, Acta Derre. Venereol., 79, 366-369 (1999). (12) K. Tsukahara, Y. Takema, S. Moriwaki, T. Fujimura, and G. Imokawa, Derreal fluid translocation is an important determinant of the diurnal variation in human skin thickness, Br. J. Dermatol. (in press). (13) K. Tsukahara, Y. Takema, S. Moriwaki, T. Fujimura, T. Kitahara, and G. Imokawa, Age-related alterations of echogenicity in Japanese skin, Dermatology, 200, 303-307 (2000). (14) M. Gniadecka, J. Serup, and J. Sondergaard, Age-related diurnal changes of dermal oedema: Evaluation by high-frequency ultrasound, Br. J. DermatoL, 131, 849-855 (1994).
j. Cosmet. Sci., 52, 399-405 (November/December 2001) Simultaneous quantitative determination of fluorine and sodium monofluorophosphate in oral hygiene products LAI-HAO WANG, Department of Applied Chemistry, Chia Nan University of Pharmacy and Science, Tainan, Taiwan 7171 O, R.O.C. Accepted for publication August 15, 2001. Synopsis An ion chromatographic method for simultaneous quantitative determination of fluorine and sodium monofluorophosphate in oral hygiene products is described. The liquid chromatographic system consisted of an IC A1 polymethacrylate-based anion exchanger and carbonate buffer (pH 9.85) as the mobile phase with a conductive detector. Various excipient ions were investigated with respect to their interference with the determination of fluoride. Comparison with results obtained from a fluoride-ion electrode technique show good agreement. INTRODUCTION Epidemiologic and experimental evidence concerned with the relationship of fluoride to dental caries suggests that fluoride solutions applied to the external surfaces of teeth may decrease their susceptibility to caries. The first two papers on the effect of topical application of fluorides were made by Bibby in 1942 (1) and Chenye in 1946 (2). Fluorine derivatives including sodium fluoride, stannous fluoride, and sodium mono- fluorophosphate (MFP, Na2PO3F) are incorporated into dentifrices or mouthwashes as chemotherapeutic agents (3). However, fluoride is absorbed into the blood from the gastrointestinal tract. It is then mostly deposited in bone or excreted in urine (4). The acute and chronic toxicity values (LD5o values in the rat and mouse) of sodium fluoride, stannous fluoride, and MFP have been investigated. The toxicity of stannous fluoride is similar to that of sodium fluoride. Stannous fluoride and sodium fluoride are more toxic than MFP by factors of 1.4-3.0 (4). The Food and Drug Administration (FDA) has promulgated regulations for safe and effective oral hygiene products. Active anticaries agents in dentifrices were 0.22%, 0.40% and 0.76% for sodium fluoride, stannous fluoride, and MFP, respectively. The final product, containing 0.02% fluoride ion in oral rinses, was based on in vitro data as well as on clinical trials (4). A mutagenicity study on three fluorine derivatives selected from the cosmetic guidelines 399
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)