ANTIBACTERIAL SUBSTANTIVITY 371 APPLICATION OF TEST PRODUCTS In each study, assignment of the test and placebo soaps to the subjects was made according to a computer-generated randomization. Following the 7-14-day washout period, subjects performed a supervised wash of their forearms three (3) times per day at the laboratory with their assigned bar of soap. Washes were at least one hour apart. To assess immediate residual effectiveness after washing, subjects performed seven washes: three washes on days 1 and 2 and one wash on day 3 of the test period. For evaluation of prolonged residual effectiveness, subjects performed nine washes: three washes on days 1, 2, and 3. Subjects were permitted to shower and bathe during the test period, using a non-antibacterial bar soap. However, they were instructed not to wash their arms. Showering and bathing was restricted after the last wash. Subjects were permitted to use a non-medicated lotion on their forearms whenever the forearms became dry, except after the final wash. At each wash visit, the subjects were closely supervised as they washed their own arms. Each forearm was washed separately with the appropriate soap. The volar surface of the forearm and the bar soap were wetted under running tap water maintained at 95 ø- 100øF. The bar was then rubbed on the forearm using an up-and-down motion from the wrist to the elbow for 15 seconds. The lather was then rubbed on the forearm using the hands in the same motion for another 45 seconds, followed by a 15-second rinse. Each forearm was patted dry with a paper towel. INOCULATION OF TEST SITES On the last day of the test period (immediately after the seventh wash or 24 hours after the ninth wash), three (3) circular test sites, 3.0 cm in diameter, were marked on the volar surface of each forearm. These circular sites were evenly spaced along the midsec- tion of each forearm, avoiding the area on the wrist and the elbow crease. Each test site was inoculated with S. a•reus, strain 502A (ATCC # 27217) that had been grown at 35 ø + 2øC for 20 5 2 hours in trypticase soy broth (TSB). For inoculation, a ten-fold dilution of the S. aureus broth culture was made, and 10 lal of the diluted bacterial culture was applied to each test site. The inoculum concentration ranged between 3.4 x 105 and 7.8 x 105 colony-forming units (CFUs) for the inoculation immediately fol- lowing the final wash and between 1.2 x 10 6 and 1.5 x 10 6 CFUs for the inoculation 24 hours following the final wash. Using a sterile inoculating loop, the inoculum was spread within the center of the test site while remaining 4 to 5 mm from the marked edge. Each inoculated test site was immediately occluded with a 25-mm Hill Top Chamber © without the non-woven absorbent pad. The chambers were secured to the skin with an adhesive dressing (Durapore ©, 3M). Sites remained occluded for intervals of 30 minutes, two hours, or five hours. HARVESTING OF SURVIVING ORGANISMS At the completion of each occlusion interval, the chambers were removed and the sites were sampled for surviving organisms using the cup scrub technique (1,2). The sampling
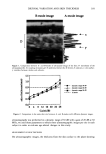
372 JOURNAL OF COSMETIC SCIENCE solution consisted of 0.075 M phosphate buffer with 0.1% Triton X-100 detergent at pH 7.9 and letheen as the neutralizer. Letheen has been previously validated and long used in this laboratory as a suitable neutralizer for formulations containing TCC. The samples were serially diluted in half-strength (0.0375 M) buffer in ten-fold dilutions to 10- . Aliquots (0.1 ml) of each undiluted and diluted specimen were spread in triplicate on the surface of trypticase soy agar with 5% sheep blood (TSA/B). All plates were incubated aerobically for 48 + 4 hours at 35 ø + 2øC. The number of CFUs per dilution was determined. RESULTS In both studies, the surviving CFUs of bacteria for each subject were enumerated and these values were 1og•o transformed. The mean loglo CFU counts for both the immediate and prolonged (24-hour) efficacy studies are presented in Table I. The difference in the loglo CFU counts between the antibacterial bar soap and the placebo are also shown in this table. The log•o CFU counts were compared using a Wilcoxon signed-rank test to estimate which of the bars (antibacterial or placebo) had the greatest antibacterial activity. A binomial sign test was also used to determine any significant differences between the number of subjects experiencing a reduction in CFUs with the antibacterial bar soap relative to the bar soap vehicle. The antibacterial soap was significantly (p • 0.05) more effective than the vehicle at controlling the growth of S. aureus on the skin at the 30-minute, two-hour, and five-hour occlusion times (Table I). Even when the challenge was 24 hours after the final wash, the residual benefit of the antibacterial soap was significantly (p • 0.05) more effective than that observed immediately after use. The binomial data for subjects with fewer CFUs is presented in Table II. A significantly (p • 0.05) greater number of subjects had fewer surviving organisms on the arm washed with the antibacterial soap than on the arm washed with the placebo soap at all contact times. This pattern continued even 24 hours after the final wash. DISCUSSION Washing with either an antibacterial soap or with a non-antibacterial soap will remove bacteria from the skin due to the surfactancy of the soap base and the mechanical action of the wash procedure. Still, washing is often incomplete, and the surviving bacteria can readily recover. However, antibacterial soaps are generally formulated to deposit an antimicrobial agent on the skin that may remain effective for an extended period after washing. As demonstrated in this work, this residual efficacy can inhibit the growth of organisms that survive on the skin or come in contact with the skin subsequent to washing. Within two to five hours after contact with bacteria, individuals who had recently washed exhibited greater than 1 log difference between the antibacterial soap and the soap vehicle. These results are consistent with those reported by Finkey eta/. (9) and Scala et aL (10), in which they observed a residual difference of approximately 1.3 logs and 2.0 logs, respectively, between an antibacterial soap containing 1.5% TCC and
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)