2001 ANNUAL SCIENTIFIC SEMINAR 409 Sunscreen/Skin Protectant: While this product crosses the lines of two FDA monographs, the FDA has said that this is a reasonable and proper combination product that has enjoyed popularity for many years. Sunscreen/Moisturizer: Many companies have marketed products of this type for several decades and consumers have come to almost expect sunscreen protection from their moisturizer products. Quite often the difficulty comes in as regards improper labeling of these products. When a cosmetic claim is made for a sunscreen product, combination drug and cosmetic labeling must be adhered to. The sunscreens must be listed separately using their drug designations and the other ingredients must be listed in decreasing order by % w/w using their INCI designations. How many of us have seen products of this type list the water as "purified water" i.e.: drug water? Which is clearly incorrect! Sunscreen/DHA: This combination product is a most interesting one. The FDA has said that if one used DHA and refers to "tanning", which of course has nothing at all to do with the action of DHA products, and does not incorporate a sunscreen or claim an SPF, then a warning statement must be displayed informing the consumer that "this product does not contain a sunscreen" and does not protect from the sun. This means that the FDA is now regulating the label of a cosmetic product and requiring a warning label because it does not contain a drug! While this seems silly at first glance, the FDA believes (and they are correct) that consumers who purchase DHA self "tanning" products expect that they will be protected (to some unknown extent) by having their skin "tanned" and this is essentially not true. Thus this cosmetic product makes an implied drug claim without having any drug action and would be mislabeled if it did not contain the warning statement. Sunscreen/Antiacne: There is much confusion regarding this combination product. Certainly, use of some antiacne drugs, such as tetracycline increases a person's sensitivity to UV in general and UVA in particular. However, there are some combinations that are questionable such as sunscreen and benzoyl peroxide. Sunscreen/Antioxidant: While it is commonly believed, with much supporting data, that antioxidants play a beneficial role in acting as free radical scavengers to protect the skin from the ravages of singlet oxygen, the FDA has not commented on whether this is a drug action. Keep in mind that something becomes a drug (according to the FDA) if it is intended to affect the structure or function of the body/skin or is to be used in the cure, diagnosis or mitigation of a disease. Based on this definition are antioxidants drugs? I for one hope, and think, not.
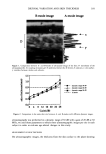
410 JOURNAL OF COSMETIC SCIENCE IMPROVMENTS IN SPF CAN BE ACHIEVED THROUGH OPTIMIZATION OF SURFACE FILM INTERACTIONS* Duncan Aust, Ph.D., Vitthal Kulkarni, Ph.D., Joretta Wong, James Wilmott and James Hayward, Ph.D. The Collaborative Group, Ltd., 3 Technology Drive, East Setauket, NY 11733 vitthal. kulkarni @ collabo. com INTRODUCTION Effectiveness of a sunscreen is judged by the sun protection factor (SPF) assigned to it (Cole, 2001). It is well known that depending on the emulsion system and the reproducibility of that system, the same concentrauon of sunscreen agents may produce a wide array of final SPFs. We have consistently produced organic sunscreens using our proprietary high-pressure high-shear technology that have well characterizable nano-particles. Our studies suggest that two fold increase in SPF can be achieved using the nano-dispersions of sunscreens compared to conventional emulsion systems indicating that particle size of the dispersion plays significant role in providing sun protection. MATERIALS AND METHODS A surfactant-free nano-dispersion of sunscreen containing 12% butyl methoxydibenzoylmethane (parsol 1789 La Roche) and 30% of ethykhexyl methoxyciunamate (OMC ISP Van Dyk) was prepared using our proprietary high-pressure high-shear technology and used it at 25% level to produce a finished product. A conven6onal sunscreen containing the same amounts of the organic sunscreen agents was prepared to compare the properties of finished products. In-vivo SPF was determined on a human panel in accordance with the federal regulations. Particle size was measured using Zetasizer 3000 (Malvern Instruments, Inc.). To test the effect of bright sunlight on the sunscreen agents, the two samples (lotion prepared using the nano-dispersion and a conventional lotion) were drawn on glass slides and exposed to natural bright sunlight for a desired period. Active ingredients in the sunscreens were then quantitatively analyzed via HPLC (Jiang et al. 1996). Quality of the film formed by the two sunscreen products, upon rubbing on skin, was studied using a Environmental Scanning Electron Microscope (ESEM) (FeiCo- Phillips, FEG XL-30). The known amounts of sunscreen products were applied on in-vitro skin (IMS, Inc.) and spread on equal area using a finger covered with a finger cot and examined in ESEM. RESULTS AND DISCUSSION Figure 1A shows the in-vivo SPF values of the two sunscreen products. Lotion made with nano- dispersion showed SPF of 15 while the lotion prepared in a conventional manner showed SPF of 8.1 suggesting that particle size, population homogeneity, and spreading behavior may have a significant influence on the ability of the formulated sunscreen actives to protect against the UV radiation. o Figure 1: A: In-vivo SPF for lotions prepared using our proprietary surfactant-fiee nano-dispersion of sunscreens and the 1o6on prepared in a conventional manner •at contained same level of sunscreen actives. B: Average particle size of the two lotions. Particle size of the lo6on made using a nano-dispersion (500-1000 nm, poly index =0.12) is much smaller than that made in a conventional manner (1000 - 3000 nm poly index =1). Size distribution showed that lotion with nano-dispersion had a homogeneous population while the conventional lotion showed poly of 1 suggesting that two or more populations with large difference in size. The particle size of nano-dispersion (the raw material) is 300-600 nm suggesting that no significant change in the particle size occurred for the lotion made from a nano-dispersion. Figure 2 shows the surface of the in-vitro skin model
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)