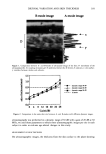
2001 ANNUAL SCIENTIFIC SEMINAR 421 STRUCTURE AND PROPERTY RELATIONSHIP OF NONIONIC SURFACTANTS AND EMULSIFIERS Silke Hoppe, Ph.D. SASOL, Austin, TX INTRODUCTION The choice of an emulsifier or surfactant is of vital importance to the preparation of stable cosmetically elegant products, especially when formulations contain hard to emulsify or solubilize ingredients like silicones, lactates etc. The benefits and dosage level of surfactants in hair and skin care applications are influenced by several different properties including their hydrophilic lipophilic balance (I-{LB). Alcohol Ethoxylates (AE) are the most widely used nonionic surfactants on the market today. The I-{LB of Alcohol Ethoxylates can be calculated by a common method: dividing the weight % of EO by 5. This method does not differentiate between different hydrophobe structures (multiple branched, linear, monobranched), and may lead a formulator to believe that one ethoxylate can easily be replaced for another. This study compares five series of nonionic surfactants based on structurally distinct hydrophobes of similar molecular weight. Each series covers HLB values from 8 to 15. Linear, defined I3-branched (Guerbet), multiple branched, monobranched Oxo and secondary hydrophobes with varying degrees of ethoxylation will be compared regarding their solubility, surfactant and emulsifier performance. Figure 1 illustrates the differences in alkyl chain structure of those hydrophobes. The study also discusses the effect of the introduction of propylene oxide to Guerbet alcohol based surfactants. Guerbet 12 Alcohol Tridecyl Alcohol Branched Oxo Alcohol Lauryl Alcohol •OH Secondary Alcohol • OH Figure 1. Structural representation of parent alcohols METHODS 250 -• --•-Guerbet 12 Ethoxylates I '•- Lauryl Ethoxylates 200 F "•- Tridecy' Ethøxylates / o 40 45 50 55 60 65 70 75 ß d% EO Figure 2. Wetting Data of Alcohol Ethoxylates The linear and branched ethoxylates were synthesized from the respective alcohol and ethylene oxide (EO) using a proprietary catalyst (NOVEL© II), which yields narrow ethoxylate distribution. It also effectively lowers the amount of free alcohol and suppresses unwanted PEG formation for branched hydrophobes. •,2 The secondary alcohol ethoxylates were obtained as product samples from the manufacturer. The solubility of the ethoxylate in oil was determined by making 1 and 10% solutions of the ethoxylates. Wetting times were determined by the dip-method, foaming was measured using the Schlag method. CMC and Surface Tension were measured with a tensiometer. Cloud Points were determined in water and BDG. REStILTS AND DISCUSSION Comprehensive studies on the behavior of alcohol ethoxylates depending on the number of ethylene oxide units or the length of the alkyl chain have been published in the literature, but only few data points are available regarding the influence of the alkyl chain structure on performance characteristics. 3's The physical property and performance data presented here show that the behavior of alcohol ethoxylates is influenced quite significantly by both the hydrophobe structure and the degree of ethoxylation.
422 JOURNAL OF COSMETIC SCIENCE The wetting data (Figure 2) illustrates this effect'well at HLB 11 (55 wt % EO), all ethoxylates, branched and linear, behave fairly similar with wetting times between 17 and 27 seconds. However, at HLB values 8 or 15 the differences are more significant. A lauryl ethoxylate with HLB 15 has a wetting time of 265 seconds, while a Guerbet 12 Ethoxylate has one of only 34 seconds. In general, Guerbet Ethoxylates are the fastest wetting agents, followed by tridecyl AE and secondary AE while the linear AE are the slowest wetting agents. The foaming test results followed expected trends, more EO provides more foam, a Guerbet 12 AE foams considerably less than linear and tridecyl AE. Cloud Points differ also significantly, which is important for emulsification temperature, since the Cloud Point is essentially the temperature at which the ethoxylate becomes oil soluble. The differences can not be attributed completely to the amount of free alcohol present and must therefore depend on the hydrophobe structure as well. Figures 3 and 4 present the data for critical micelle concentration (CMC) and equilibrium surface tension. These examples show that the surface activity depends greatly on the hydrophobe structure, which complements literature reports on the influence of the alkyl chain length on CMC. 7 While lauryl ethoxylates have the lowest CMC, they are the least effective in lowering the surface tension. Guerbet 12 ethoxylates have the highest CMC, but are very effective in lowering the surface tension. Further investigations regarding micelie shape and size are necessary to explain this phenomenon more clearly. This data helps to determine which surfactant is best for a particular formulation - if the intent for using an ethoxylate is to have a large amount of monomer surfactant present without micellization, the Guerbet ethoxylates should be used. For applications where a small amount of surfactant is meant to yield micelies, the linear lauryl ethoxylates can be recommended. :500- --,- Guerbet 12 AE _ --- Lauryl AE -- -*- Tridecyl AE _ --- Secondary AE • Guerbet 12 AE • --- Lauryl AE --*- Tridecyl AE -..- Secondary AE 45 50 5,5 w•, E060 65 70 75 80 65 70 75 37 E 33 '•' 31 29 27 25 40 45 50 55 • E•O Figure 4. Surface Tension at CMC Figure 3. Critical Micelie Concentration Furthermore, a new set of emulsifiers will be introduced - Guerbet 12 Ethoxylates, Propoxylates and Alkoxylates (EO/PO and PO/EO block polymers), which give the formulator access to a matrix of compatible high and low HLB emulsifiers. They can be used to emulsify a variety of esters and silicone oils. For instance a Guerbet 12 PO/EO Block Polymer will emulsify and thicken a dimethicone, and a Guerbet 12 Propoxylate will dissolve in cyclomethicone and stabilize a water in cyclomethicone emulsion. Solubility and emulsifying behavior of this set of nonionics will be discussed in more detail. These surfactants are liquid over a wider range of temperatures, can improve the skin feel of the formulation and show increased effectiveness as emulsifiers over other surfactants. REFERENCES 1) Cox, M., Weerasooriya, U., J. Surfact. Deterg., 2, 59, (1999). 2) Cox, M., JAOCS, 67, 599 (1990). 3) Cox, M., JAOCS, 66, 367 (1989). 4) Rosen, M. et al., J. Phys. Chem., 86, 541, (1982). 5) Rakatuni, K. et al., "Design and selection of performance surfactants", pp. 216-247, CRC Press (1999). 6) Meerbote, M., Koch, B., Tenside Surf. Det., 31, 39, (1994). 7) J/3nsson, B. et al. "Surfactants and Polymers in Aqueous Solution" John Wiley & Sons (1998).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)