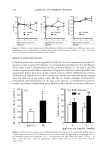
LIPOIC ACID STABILITY 459 120 100 80 •= 60 4O 0 I ?--Vitamin I?_Li_p•ø•i• acid• 50 1 O0 time (days) 150 200 Figure 10. Percentage of lipoic acid, vitamin A, and vitamin E vs time in system 4. 120 1 oo 80 õ 6o 4O [-*-- Lipoic acid I 0 50 1 O0 150 200 time (days) Figure I I. Percentage of lipoic acid vs time in system 1. 120 100 80 60 40 ipoic acid 0 50 1 O0 150 200 time (days) Figure 12. Percentage of lipoic acid and vitamin E vs time in system 3. their rheological behavior in relation to the base emulsion used as control (Figure 2). After six months of storage at room temperature, no significant variations in viscosity or thixotropy were observed in any of the systems under study since its elaboration. pH variations in the same period are indicated in Table II. Forced degradation of vitamins A and E and lipoic acid were performed to study the stability of the formulations and
460 JOURNAL OF COSMETIC SCIENCE the specificity of the methods (Tables III-V). Degradation was observed in the stressed sample as a decrease in the level of the active drug and increased levels of the degradation products. No interfering peaks at the retention times of the drugs were observed in any of the stressed samples (Figures 3-5) Typical chromatograms of the formulations can be seen in Figures 6-8 The contents of lipoic acid in the formulations containing vitamin A remained stable during the first four months (Figures 9 and 10, formulations 2 and 4). In the presence of vitamin E, vitamin A is more stable (Figure 10). Formulations 1 and 3 lost almost 30% of lipoic acid activity within the first 30 days (Figures 11 and 12). Vitamin E remained practi- cally stable during the study period (Figures 10 and 12). CONCLUSIONS Based on experimental results, we conclude that although lipoic acid is not very stable in these formulations, the presence of vitamin A favors its chemical stability. Further- more, vitamin E could not prevent lipoic acid from degradation, although it was stable during the study. These findings contribute to a new perspective in the use of the vitamin A as an antioxidant in association with sulfur compounds. As in the case ofpolyunsaturated fatty acids, vitamin A could react with thiyl radicals and regenerate the thiol. In order to achieve a longer useful life of this drug in cosmetic emulsions such as those we have studied, it is necessary to have a better stabilization of vitamin A. ACKNOWLEDGMENTS This work was supported by grant B005 given to A. I. S. by the University of Buenos Aires. The authors acknowledge the support of Merck Qufmica Argentina, which do- nated the LiChrosphere © 100 RP-18 (512m) 125-4 column. We are also grateful for the assistance provided by Craveri S.A.I.C. and Concolor S.A. REFERENCES (1) A.L. Lehninger, "Vitamins y Coenzymes," in Biochemistry, 2nd ed. (McGraw Hill Interamericana, 1998), pp. 353-354. (2) P. Morganti, C. Bruno, F. Guarneri, A. Cardillo, P. Del Ciotto, and F. Valenzano, Role of topical and nutritional supplement to modify the oxidative stress, Int. J. Cosmet. Sci., 24, 331-339 (2002). (3) S. Richerr, A. Schrader, and K. Schrader, Transdermal delivery of two antioxidants from different cosmetic formulations, Int. J. Cosmet. Sci., 25, 5-13 (2003). (4) L. Packer, E. W. Witt, and H.J. Tritschler, Alpha-lipoic acid as a biological antioxidant, Free Rad. Biol. Med., 19, 227-250 (1995). (5) K. Marangon, S. Devaraj, O. Tirosh, L. Packer, and I. Jialal, Comparison of the effect of tx-lipoic acid and tx-tocopherol supplementation on measures of oxidative stress, Free Rad. Biol. Med., 27, 1114- 1121 (1999). (6) S. K. Jain and G. Lira, Lipoic acid decreases lipid peroxidation and protein glycosylation and increases (Na + + K +) and Ca++-atpase activities in high-glucose-treated human erythrocytes, Free Rad. Biol. Med., 29, 1122-1128 (2000). (7) C. Chatgilialoglu, L. Zambonin, A. Altieri, C. Ferreri, Q. G. Mulazzani, and L. Landi, Geometrical
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)