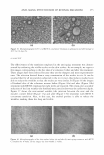
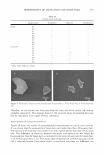
ANTI-AGING EFFECTIVENESS OF RETINOL EMULSIONS 275 (a) (b) Figure 15. Microphotographs ( 12 5 0 x) of RETI C concentrate emulsion at opening (a) and after storage at 40 ° C for 10 days (b). IN VIVO TEST The effectiveness of the emulsions employed in the anti-aging treatment was demon strated by evaluating the visible results on the skin surface. As an example, we report a few images corresponding to the face skin of a volunteer, before and after the treatment. These images have been selected because they are the sharpest and most representative ones. The selection derived from a wary examination of the results in vivo. It can be assumed that for all ten panelists subjected to the in vivo test, the products studied were able to reduce the wrinkles so that the results are very similar. In Figure 16 the images of forehead skin of the volunteer woman before (Figure 16a) and after (Figure 166) treatment with RETI C emulsion for eight weeks are reported. The cream gives a notable reduction of the lion wrinkle (the forehead-nose junction between the eyebrows) depth. Figure 17 shows the nose-mental wrinkle (the junction between the nose and the mouth's corner) before (Figure 17a) and after (Figure 176) treatment with RETI C concentrate emulsion. Also, in this case, the studied product is able to reduce the wrinkles, making them less deep and visible. (a) (b) Figure 16. Microphotographs of the skin surface before (a) and after (b) anti-aging treatment with RETI C emulsion for 8 weeks. In the images the lion wrinkle is visible.
276 JOURNAL OF COSMETIC SCIENCE (a) (b) Figure 17. Microphotographs of the skin surface before (a) and after (b) anti-aging treatment with RETI C concentrate emulsion for 8 weeks. The images show the nose-mental wrinkle. CONCLUSIONS RETI C and RETI C concentrate emulsions have been shown to be stable under usual employment conditions. Nevertheless, the vitamin A contained in these creams de graded under UVA and UVB irradiation. In drastic conditions, according to the rheo logical studies performed after storage at 40 ° C, both RETI C and RETI C concentrate emulsions show only a limited loss of viscosity linked to a slight degradation of the vehicle. In contrast, retinal concentration decreased more quickly after storage at 40 ° C than after storage at 25 ° C. Therefore, in this case, vehicles cannot be considered alter native substrates of degradation compared to retinal. RETI C and RETI C concentrate emulsions are declared W /0/W emulsions, but they are rather unstable, as shown by optical microscopic analysis over time. It would be useful to improve the physical stability of these two commercial products in order to obtain a higher stability of active ingredients. In fact, the structure of the W/0/W emulsions could be farther stabilized against degradation by ascorbic acid, and consequently it could improve the stability of retinal, whose degradation can be promoted by the instability of vitamin C. Moreover, retinal stability could be enhanced by employing innovative formulation technology like encapsulating it in SLN. The stability assays discussed in this paper (viscosity, rheology, conductivity, optical microscopy, and the UV irradiation test) have been reported in the general literature ( 13, 14). These procedures have significant results, as they allow one to predict efficiently the behavior of commercial products. On the basis of the in vivo test, RETI C and RETI C concentrate emulsions can be considered effective in anti-aging treatments, reducing progressively the appearance and the depth of wrinkles. REFERENCES (1) P. T. Pugliese and C. B. Lampley, Biochemical assessment of the anti-aging effects of cosmetic prod ucts,]. Appl. Cosmetol., 3, 129-138 (1985). (2) M. Galesso, M. Gatta, and F. Galiano, Comparative studies on the stability of ascorbic acid and its derivatives in various mattixes and interaction with commonly used cosmetic preservatives, Cosmet. Toiletr. Ed. Ital., 2, 58-74 (1993). (3) R. Austria, A. Semenzato, and A. Bettero, Stability of vitamin C derivatives in solution and topical formulations,]. Pharm. Biomed. Anal., 15, 795-801 (1997).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)