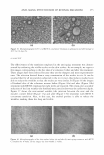
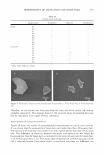
EFFICACY AND SAFETY OF DEOXYARBUTIN 303 inhibition of the melanin present in the epidermis (Table III). The lightening of grafting-induced hyperpigmentation was visibly apparent (Figure 6a-f). In addition, two of three mice treated with HQ developed brown coloration of the hair around the grafts not observed in mice treated with the vehicle or dA (Figure 6g-i). H&E stained sections for grafts treated with dA and HQ for eight weeks demonstrated no signs of inflammation or abnormal morphology (data not shown). CLINICAL TRIAL RESULTS The effect of dA and HQ on the lightening of tanned skin was compared in a clinical trial as described in the Materials and Method section. After a seven-day regimen of tanning, areas of skin were left untreated or treated blindly with either dA or HQ, three times per week for five weeks. At the end of this period, the percent of tan remaining in the untreated site was 44.6%. In comparison, the percent of tan remaining in the sites treated with either dA or HQ was 37.3% (a significant increase in tanning loss over control) versus 5 J 6% (a sigoificaor decrease in tanning loss over control), respectively. These results suggest that dA accelerated the fading of UV-induced tan, whereas HQ impeded this response. DISCUSSION Toxicity of phenolic compounds can arise from three possible mechanisms: (a) suscep tibility of agents to extracellular auto-oxidation, (b) cellular permeability, and (c) in tracellular oxidation by tyrosinase and/or other metabolic enzymes (25-27). Deoxyar butin (dA), was shown to reversibly reduce tyrosinase activity and melanin content and demonstrated less cytotoxicity, as compared to HQ, in the three normal human skin cell types (i.e., melanocytes, keratinocytes, and fibroblasts). Studies have previously demonstrated that a major component in the toxicity of phenolic compounds is attributable to reactive oxygen species produced outside the cells (2 5 ). Specifically, hydroquinone auto-oxidation was shown to occur predominantly in the extracellular environment and to be a causative event for quinone-induced cytotoxicity (27). Thus, the lower cytotoxicity of dA compared to HQ may be due to its enhanced stability and reduced auto-oxidation. The enhanced stability could be attributed to the presence of an acetyl bond in its structure that allows stability in basic conditions. In addition, and in contrast to HQ, the OH group in the para position forming the acetyl bond in dA would result in conferring dA less susceptible to auto-oxidation. Table III Percent Melanin per Epidermal Area in Xenografts Treated for Eight Weeks with Vehicle or Various Tyrosinase Inhibirors Vehicle dA HQ TBP % Melanin/epidermal area 0.088 ± 0.07 0.061 ± 0.06 0.043 ± 0.05 0.015 ± 0.02
304 dA HQ 4-TBP JOURNAL OF COSMETIC SCIENCE Pretreatment \1-� · ·?"'':� ...._ -- !"'I- _,._.. 8 Wk treatment Figure 6. Grafting-induced hyperpigmentation is reduced by dA. Grafts described in Figure 5, at the onset of the treatment (a,c,e) and after eight weeks of treatment (b,d,f) with dA (a,b), HQ (c,d), or TBP (e,f), demonstrate the skin-lightening effect of each tyrosinase inhibitor. In addition, grafts treated for eight weeks with vehicle only (g) as opposed to dA (h) or HQ (i) exhibit darker versus lighter pigmentation, respectively. Note that in two of the three HQ-treated grafts a brown stain coloration adjacent to murine hair (arrows in i) is observed. The relative cellular permeability of phenols may also correlate with the effect of the compound on cellular viability, as well as its efficacy as its tyrosinase inhibitor (25). Relatively non-polar phenols, like dA and HQ, are more permeable and thus have the potential to be more toxic as compared to more polar phenols, like AR, that less readily enter the cell (25). Our present analysis demonstrates that AR is a relatively safe compound compared to dA and HQ. However, AR is either an ineffective tyrosinase inhibitor or promotes tyrosinase activity in light-versus-dark human melanocytes, re spectively. The ineffectiveness of AR as a tyrosinase inhibitory agent is in agreement with the findings of Curto et al. ( 15 ). However, an increase in pigmentation by AR has been previously reported in several studies (19,20). In contrast, several studies have demonstrated that AR is a tyrosinase inhibitor (17-19). However, these latter studies utilized high concentrations of AR in a culture system (17,19) and/or assessed tyrosinase inhibition using cell lysate rather than intact cells (17-19). This suggests that AR, although an effective inhibitor of the catalytic activity of isolated tyrosinase, is relatively
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)