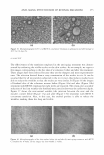
J. Cosmet. Sci., 57, 291-308 Quly/August 2006) Comparative efficacy and safety of deoxyarbutin, a new tyrosinase-inhibiting agent SAJA H. HAMED, PENKANOK SRIWIRIYANONT, MITCHELL A. deLONG, MARTY 0. VISSCHER, R. RANDALL WICKETT, and RAYMOND E. BOISSY, University of Cincinnati College of Pharmacy (S.H.H.J R.R. W.)J Skin Sciences lnstituteJ Cincinnati Children's Hospital Medical Center (P.S.J M.0. V. J R.E.B.)J Department of ChemistryJ University of Cincinnati (M.A. d. L.) and Department of Dermatology, University of Cincinnati College of Medicine (S.H.H., R.E.B.)J Cincinnati, OH 45267. Accepted for publication March 22, 2006. Synopsis Several tyrosinase inhibitors have been developed and utilized to ameliorate various cutaneous hyperpig mentary disorders and complexion discolorations. Deoxyarbutin (dA) (i.e., 4-[(tetrahydro-2H-pyran-2- yl)oxy }phenol), designed using quantitative structure-activity relationships (QSAR), demonstrates effective inhibition of mushroom tyrosinase and skin-lightening capability (1). However, its comparative safety, effectiveness, and reversibility to other known tyrosinase inhibitors in human melanocytes had not been determined. The effect of dA was assessed in cultured human skin cells, on xenographs, and with a clinical trial. Using cultured human melanocytes, the maximum concentration of dA that allowed 95% viability was fourfold greater than for hydroquinone (HQ), indicating that dA is less cytotoxic/cytostatic than HQ. The viability of cultured human keratinocytes and fibroblasts was also less compromised by increasing concen trations of dA as opposed to HQ. At the maximum concentration allowing normal cellular viability, dA effectively inhibited tyrosinase activity and melanin content in human melanocytes, whereas HQ was marginally inhibitory. Upon removal of dA, tyrosinase activity and melanin content was normalized within five days. Topical application of dA on human xenografts resulted in a gradual and visually apparent skin lightening effect during an eight-week period. In a clinical trial, dA facilitated fading of pre-tanned skin to a statistically significant greater extent than either HQ or no treatment. These results demonstrate that dA is a potentially saf�, effective, and reversible tyrosinase inhibitor. INTRODUCTION Melanin in an excessive amount and/or altered disposition is the basis for a number of congenital and acquired hyperpigmented skin diseases (2). Hyperpigmentation diseases also manifest psychosocial and cosmetic problems since they are common on sun-exposed Address all correspondence to Raymond E. Boissy. 291
292 JOURNAL OF COSMETIC SCIENCE areas of the face and the neck (3,4). In addition, maintaining a uniform and lighter basal skin tone is a global concern that is a result of personal preferences and/or cultural biases. Due to the therapeutics and socio-economic importance of altering skin pigmentation, many efforts have been made to develop and recognize compounds that act as depig menting agents. Various remedies are available in the market, but none are completely satisfactory (4). Therefore, the development of an effective, controllable, and safe agent to regulate melanin synthesis in the skin is of great interest to medical practitioners and their patients. As a result of the key role played by tyrosinase in melanin biosynthesis, most popular skin depigmenting products use a tyrosinase inhibitor as an active ingredient (e.g., hydroquinone, kojic acid, arbutin). The success of treatment using these products is limited for two basic reasons: safety or overall effectiveness. Hydroquinone (HQ) has been banned in cosmetic use in Europe and is currently available only by prescription. It is considered a melanocyte cytotoxic agent as a consequence of its oxidation, through tyrosinase and/or spontaneously, to highly reactive species such as hydroxybenzoquinone and p-benzoquinone toxic products capable of disrupting fundamental cellular processes (4-7). Kojic acid (KA), an inhibitor of tyrosinase, has high sensitizing potential (8,9) and recently has been banned in Japan because of mutagenecity concerns (10). The effectiveness of KA has been demonstrated both in vitro (11,12) and in clinical trials (13,14). However, a relatively recent study demonstrated that KA treatment is not associated with reduction in pigmentation, using a cell-based assay (15 ). Arbutin (AR) has been traditionally used in Japan to treat pigmentary disorders (16,17), but its effectiveness is controversial. While several studies have demonstrated that arbutin can inhibit tyrosinase activity in cultured human melanocytes (17 ,18), many studies have demonstrated that arbutin increases pigmentation (19,20), or ineffectively inhibits ty rosinase activity both in the cell-free and cell-based assays (15 ). Because of the need to provide a skin depigmenting agent that is more efficacious, more stable, and less cytotoxic than the available skin-depigmenting agents, a novel com pound, deoxyarbutin (dA) 4-{(tetrahydro-2H-pyran-2-yl)oxy }phenol, was developed us ing quantitative structure-activity relationships (QSAR) of tyrosinase inhibitors (1). In this report, we analyze the safety, effectiveness, and reversibility of dA on human melanocytes in comparison to the aforementioned tyrosinase inhibitors, hydroquinone, kojic acid, and arbutin. In addition, we demonstrate the skin lightening effect of dA on human skin. MATERIALS AND METHODS CHEMICALS AND SOL VENTS Hydroquinone (HQ), kojic acid (KA), arbutin (AR), and 4-tertiary butylphenol (TBP) were purchased from Sigma Chemical (St. Louis, MO). Deoxyarbutin (dA) was initially synthesized by the authors and scaled up by Girindus America Inc. (Cincinnati, OH). For culture studies, dA, HQ, KA, and TBP were dissolved in sterile DMSO, while AR was dissolved in MCDB 15 3 media (Irvine Scientific, Santa Ana, CA). TBP was included in this comparative study because it is a tyrosinase inhibitor implicated in contact/ occupational vitiligo (21). Stock solutions of compounds were prepared and protected
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)