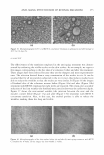
284 JOURNAL OF COSMETIC SCIENCE HO HO�oH o=c HO b OH HO �c-o�Q }-i-oH Y-8 �o � HO OH I OH Ao HOYoH OH COOH HO OH OH Compound 1 Compound 2 Figure 1. Chemical structures of compounds 1 and 2 isolated from M. heterophylla. 110.31-146.07 ppm in the 13 C-NMR spectrum. The final structure was identified as 3,4,5-trihydroxybenzoic acid (Figure 1) by referring to the reported data (17). We studied the antioxidant effects of two compounds from M. heterophylla using DPPH and superoxide radicals. These compounds were found to have a strong antioxidant activity. Compounds 1 and 2 exhibited a potent scavenging activity against the DPPH radicals, with SC 50 (the concentration of the sample required for 50% of the free radicals to be scavenged) values of 3.9 µM and 13.3 µM, respectively. Furthermore, compound 1 appeared to be the most efficient in comparison with the three reference compounds, vitamin C (SC 50 60.0 µM), vitamin E (SC 50 30.0 µM), and epigallocatechin-3-gallate (EGCG, SC 50 6.4 µM), which is well known as a scavenger of DPPH radicals (Table I) (18,19). Also, compounds 1 and 2 exhibited a potent scavenging activity against the superoxide radicals in the xanthine/xanthine oxidase system, with SC 50 values of 4.3 µM and 4.0 µM, respectively, compared with butylated hydroxyanisole (BHA, SC 50 Table I Antioxidant Effects of Compounds 1 and 2 Isolated from M. heterophylla SC50a values (µM) Compounds DPPH6 Superoxide anion C 3.9 4.3 2 13.3 4 Vitamin C* 60 NDd Vitamin E* 30 NDd EGCG* 6.4 4.9 BHA* NDd 180 a Concentration giving a 50% decrease of DPPH and superoxide radicals. The values are the means of triplicate experiments with SD. 6 1,1-diphenyl-2-picrylhydrazyl radical. c Superoxide anion radicals were produced from xanthine/xanthine oxidase oxidation system. cl Not determined. * Used as a positive control.
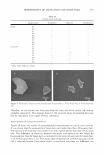
M. HETEROPHYLLA AND INHIBITION OF MMP-1 285 180.0 µM) and EGCG (SC 50 4.9 µM), which is well known as a scavenger of superoxide radicals (Table I) (20-22). MMPs and ROS play a major role in skin aging. In fact, skin aging characterized by severe connective tissue degradation has been linked to induced synthesis and activity of different members of the matrix-degrading metalloprotease family (23). Recent data suggest that UVA irradiation influences gene expression of MMP-1 and TIMP-1 and the protein expression of MMP-1,2 and TIMP-1,2 in human dermal fibroblasts, as well as the enzyme activity of MMPs (23,24). Hantke et al. (24) have reported that flavonoids and vitamins reduce MMP expression at the protein level and MMP activity in the skin after sun exposure. We first studied the inhibitory effect of these compounds isolated from M. heterophylla on in vitro collagenase activity. As a control, 1, 10-phenanthroline, which is well known as the metal chelator and general inhibitor of MMPs, was used to optimize conditions for screening potential gelatinase or collagenase inhibitors (25 ). Compounds 1 and 2 were found to have a potent and distinct inhibitory activity against MMP-1, with IC 50 values of 60.0 µM and 100.0 µM, respectively (Figure 2). These compounds inhibited MMP-1 activity in a dose-dependent manner. Especially, compound 1 showed a twofold higher inhibitory effect for MMP-1 activity than that of 1,10-phenanthroline (121.0 µM). The effect of these compounds on the viability of HDFs was investigated for photopro tective activities on mRNA and protein level by the MTT test. These compounds did not show cytotoxicity against HDFs in the test dose as compared to the control (Figure 3 ). 100 8=� 80 u � 0 I L. a.. +-' 60 � C 0 � u ..... ..... 0 0 40 C � 0 .c 20 .c C 0 500 400 100 10 Concentration (µM) Figure 2. Inhibitory effects of compounds 1 and 2 isolated from M. heterophylla on collagenase (MMP-1) activity. Fluorometic assays of the activities of MMP-1 was performed in the presence of increasing con centrations of 1, 10-phenanthroline (□), compound 1 (e), and compound 2 (0). The results are expressed as the average of triplicate experiments with SD.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)