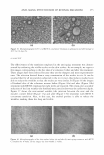
MICROEMULSIONS OF TRIGLYCERIDE-BASED OILS Type IV: Single-phase microemulsion Type I: 1continuo Type II: Reverse micelles 311 ': ': '\ '/ \\ \\\/ :·::,:,:·::::,:::··::·:::::: � Salinity Curvature ( H)= 1 /R + 0 Reducing Curvature Figure 1. Fish diagram showing phase behavior and changes in curvature with surfactant concentration and formulation hydrophobicity as adjusted by a scanning variable (salinity). vature decreases, a Type II microemulsion (W/0 microemulsion) exists. When the hydrophobicity is intermediate between these two conditions, and at lower surfactant concentration, a three-phase microemulsion or Type III microemulsion occurs, with a net zero curvature. When the surfactant concentration increases within the Type III region, a Type IV microemulsion can be obtained. The minimum surfactant concentra tion for complete solubilization of the water and the oil is where the three-phase and one-phase regions (Type IV) meet, which appears at relatively high surfactant concen trations. Fish diagrams with similar behavior have been reported elsewhere (1,5,7,9-12). For example, Jakobs et al. (9) obtained the well-known fish diagram for water-n-decane nonionic surfactant systems by plotting surfactant concentration versus the scanning variable of temperature. Von Corswant and co-workers (5 ,7) plotted fish diagrams between alcohol concentration as a function of surfactant concentrations for triglyceride microemulsification. Formation of microemulsion systems with short-chain oils or alkanes has been exten sively studied (9,13-15) a large range of surfactants and additives can be used to control their microstructural properties. However, microemulsification of triglycerides, and in particular long-chain triglycerides, is very challenging (5-8,10,16-33). These triglyc erides have minimum solubility in microemulsion systems and thus tend to form liquid crystal mesophases. This is due to the fact that the hydrocarbon chain portions of the surfactant films present at triolein-water interfaces have difficulty penetrating the large triolein molecules. Therefore, higher temperatures are required to induce sufficient disorder of the triglyceride-surfactant film, which generally contains long hydrocarbon chai�s, straight and of uniform length. This disorder permits significant amounts of
312 JOURNAL OF COSMETIC SCIENCE triolein to be solubilized in such films (34). In other words, if the surfactant films were less ordered (e.g., if the hydrocarbon chains were of nonuniform length and/or branched), solubilization of triolein would be facilitated. Addition of short-chain alcohols is known to mitigate formation of undesirable liquid crystal phases (5,7,18,19,23). Joubran and coworkers (18) have also demonstrated that the formation of triglyceride microemulsion can be achieved by incorporating sucrose and a short-chain alcohol. The synergistic interactions among the alcohol and the sucrose molecules result in the destabilization of the liquid crystalline mesophases and thus facilitate the triglyceride microemulsion formation. They have also studied a micro emulsion of soybean oil, polyxyethylene (40) sorbitanhexaoleate, and water-ethanol and found that the microemulsion formation strongly depends on temperature and that the systems require large amounts of surfactants and alcohol (19). Von Corswant and co workers (5, 7) observed the presence of lamellar liquid crystal or L c phases in long-chain triglyceride systems. They explained that the L c is destabilized by incorporating water when a short-chain alcohol is present. Adding the short-chain alcohols can increase the flexibility of the surfactant film, since the L c phase is destabilized in favor of a micro emulsion phase due to the increase of the curvature (short-chain alcohols decrease the polarity of the aqueous phase). In addition, they found that adding co-oil into micro emulsion systems helps reduce the surfactant concentration and the amount of alcohol required· to form a microemulsion (7). Huang and Lips (11) also found that microemul sification of triglycerides requires high temperatures and surfactant concentrations, :whereas Minana-Parez and coworkers (35,36) have previously reported the use of alkyl sulfates with oxyethylene and oxypropylene groups, or so-called "extended surfactants," for forming efficient microemulsions of the bicontinuous type (Type III microemulsion) with polar oil, including triglycerides however, high salt concentrations (up to 7% wt NaCl) were required. The solubilization at optimum formulation with conventional surfactants (commercial ized non-extended surfactants) has been found to reach values as high as 30 ml/ml or ml/g surfactant with short-chain alkanes, and as high as 10 ml/g with hexadecane, while it can be less than 4 ml/g with mono-chain polar oils and much less with triglyceride oils (3 5 ). Graciaa and coworkers (3 7 ,38) first introduced the lipophilic linker concept to help enhance surfactant-oil interaction and thus improve the solubilization capacity of hydrocarbon and polar oils. Lipophilic linkers, such as a long-chain alcohol, tend to segregate near the oil side of the oil-water interface, close to the tails of the surfactants (39), as depicted in Figure 2. In this schematic, the surfactant, sodium dihexyl sulfo succinate, adsorbs at the oil-water interface. The lipophilic linker, dodecanol, is shown to adsorb at the palisade layer of the interface (oil side of the surfactant layer), promoting the local order and increasing the interaction between the surfactant tail and the oil phase. In contrast to the surfactant and the lipophilic linker, sodium mono- and di methyl naphthalene sulfonate (SMDNS) is a hydrophilic linker that segregates near the water side of the oil-water interface this hydrophilic linker molecule is believed to increase the total interfacial area and the overall interaction between the surfactant layer and the aqueous phase (40). For certain oils, adding a lipophilic linker alone to the microemulsion gives limited solubilization enhancement. Acosta and coworkers (39-41) found that hydrophilic linkers can help improve solubilization ability because they allow more room for lipophilic linkers to segregate and further enhance the solubilization ability.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)