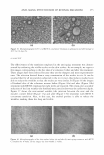
EFFICACY AND SAFETY OF DEOXY ARBUTIN 305 ineffective on intact melanocytes. The sugar residues in AR confer a significant shift in both size and polarity, e.g., the clog P of arbutin is -0.58 as compared to +0.56 for HQ, thus likely preventing it from passing through cellular membranes in sufficient con centration to inhibit tyrosinase within melanosomes. It is noteworthy to mention that, so far, the effective topical concentration of arbutin has not been formally evaluated and published (28). Once internalized, phenolic derivatives can act as substrates for tyrosinase. This requires electron donor groups (i.e., alkoxyl and hydroxyl groups) in the para position with respect to the OH group (5 ). HQ and its esters possess an electron donor group in that position. It has been demonstrated that HQ, an effective tyrosinase inhibitor (29), can be oxidized by tyrosinase to hydroxybenzoquinone and p-benzoquinone, thus generating reactive oxygen species (6,7 ,30). With dA and AR, the electron-donating ability of the para oxygen is reduced by the presence of a second oxygen ring, thus slowing or stopping the oxidation rate. Oxidation products can be produced from arbutin by mushroom tyrosinase (31). Whether dA can also be converted to quinones and generate reactive oxygen species, and at what rate, has yet to be determined. All tested tyrosinase inhibitors eventually demonstrated toxicity against keratinocytes and fibroblasts. This suggests that toxicity can arise from a non-tyrosinase-mediated mechanism. A peroxidase-mediated mechanism in the cytotoxic effects of melanogenic inhibitory agents, specifically phenolic compounds, has been demonstrated previously (32). Phenolic compounds can also serve as substrate and inhibitors for peroxidase (32-34). For example, HQ serves as a good substrate for peroxidase (35). Melanocytes, fibroblasts, and keratinocytes are all equipped with peroxidases as part of their antioxi dant enzyme system that helps the cell to counteract oxidative stress (36). A peroxidase mediated mechanism can explain the non-specific cytotoxicity of the tyrosinase inhibi tory agents we have tested. Putative depigmenting agents should subsequently lead to removal of unwanted pig ment when applied topically to skin. Thus, the effectiveness of deoxyarbutin as a treatment to reverse hyperpigmentation of human skin grafted to immunocompromised mice was assessed and compared with hydroquinone and 4-tertiary butylphenol (TBP). Xenografting of human skin onto immunocompromised mice is an excellent model for studying various aspects of skin physiology including hyperpigmentation of grafted skin on burn patients (3 7). In addition, this model is more physiologically relevant in that it allows the evaluation of melanogenic inhibitors directly on human skin. Hyperpigmen tation is common sequelae of most injuries to the skin (3 7). Farooqui et al. (3 7) showed that hyperpigmentation of human cutaneous xenografts placed on athymic nude mice was apparent as early as 4-6 weeks post-grafting. In addition, tyrosinase, a key enzyme in melanin biosynthesis, was upregulated in the grafted skin from 2 to 12 weeks post-grafting (3 7). Thus, topical application of a tyrosinase inhibitory agent could be an approach to reverse hyperpigmentation of the grafted skin and thus test its effect as a general skin-lightening modality. Topical application of dA reversed the skin hyper pigmentation of human skin grafted onto immunocompromised mice. Initial statistical analysis showed that treatments are statistically different within a week (p 0.0001). By statistically comparing the mean values for 11L * to the control group values, only one treatment group (HQ) at one time point (eight weeks) was statistically significant from the control group. Although skin lightening for dA, TBP, and even HQ at the other time points was not statistically significant from that of the control group, lightening
306 JOURNAL OF COSMETIC SCIENCE of the grafts was obvious both visually and from the gradual positive increase in '1L * compared to the vehicle-treated control. The increase in '1L * started for dA at the two-week treatment point and increased gradually over the eight-week treatment pe riod. In addition, TBP-treated grafts developed clear vitiligo-like patches. This discrep ancy may result from the high SE and low number of animals in each group. For instance, in order to be able to detect a significant difference for the dA-treated group eight weeks after treatment, using the two-sided two-sample test, we needed at least five animals in each group. The effect of dA on reversing hyperpigmentation demonstrated in the xenograft model system was recapitulated in a clinical trial. Hyperpigmentation induced by UV exposure was effectively reduced by dA. The lightening effect of dA on pathologic hyperpigmen tary disorders like melasma, solar lentigo, and post-inflammatory hyperpigmentation is yet to be determined. An interesting observation is that mice treated with HQ developed brown coloration of the albinistic hair adjacent to the grafts. Presumably this is a staining artifact from the by-products of hydroquinone oxidation. This brown pigmentation matches the reported nail plate discoloration that can develop during use of topical hydroquinone-containing cream (38--40). This color change of the nail was considered to be due to the oxidation products of hydroquinone. Hydroquinone readily oxidized to quinone and subsequently to hydroxyquinone, which is unstable and polymerizes to a brown compound. CONCLUSIONS In this report we have provided evidence that currently used skin-depigmenting agents have drawbacks in either their efficacy or their safety. Deoxyarbutin is demonstrated to be less cytotoxic than the standard skin-lightening product in the market (i.e., HQ). The cytotoxicity dA exhibited is not associated with dramatic degenerative changes in the morphology of keratinocytes as compared with HQ. Our overall goal is to provide the market with an effective skin depigmenting agent due to the fact that available depig menting agents in the market are far from satisfactory. We have clearly demonstrated that dA has the potential to be a safe and effective depigmenting agent. It also offers the potential to be an effective alternative to hydroquinone, the skin-depigmenting standard in the market with known safety drawbacks. REFERENCES (1) R. E. Boissy, M. Visscher, and M.A. deLong. DeoxyArbutin, A novel tyrosinase inhibitor with effective skin lightening potency, Exp. Dermatol., 14, 601-608 (2005). (2) J.P. Ortonne and]. J. Nordlund, Mechanisms that cause abnormal skin color, in The Pigmentary System: Physiology and Pathophysiology, J. J. Nordlund, R. E. Boissy, V. J. Hearing, R. A. King, and J. P., Ortonne, Eds. (Oxford University Press, New York, 1998), pp. 489-502. (3) A. Perez-Bernal, M.A. Mufioz-Perez, and F. Camacho, Management of facial hyperpigmentation, Am. ]. Clin. Dermatol., l, 261-268 (2000). (4) S. Briganti, E. Camera, and M. Picardo, Chemical and instrumental approaches to treat hyperpig mentation (review), Pigment Cell Res., 16, 101-110 (2003). (5) S. Passi and M. Nazzaro-Porro, Molecular basis of substrate and inhibitory specificity of tyrosinase: Phenolic compounds, Br.]. Dermatol., 104, 659-665 (1981).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)