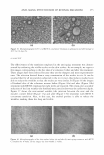
MICROEMULSIONS OF TRIGLYCERIDE-BASED OILS 315 (S*) for this composition is 0.5% NaCl. Stock solutions of AOT, hexylglucocide, and sorbitan monooleate at the selected weight ratios were prepared at different total sur factant/linker concentrations ranging from 14.19 to 56.06 wt %. The phase studies were carried out in 16 x 125 mm flat-bottomed tubes 2.5 ml of surfactant solution was added, followed by the addition of co-oil, then sebum oil. The total volume of co-oil and sebum oil required is equivalent to the amount of water present in the 2. 5 ml of surfactant solution this is to keep the WOR equal to one. The fraction of sebum oil in the oil mixtures is varied from zero (100% vol. co-oil) to one (100% vol. sebum oil). The prepared samples were gently shaken once a day for three days and left to equilibrate at room temperature (25°C) for two weeks. Phase diagrams (fish diagrams) were con structed by plotting the total surfactant/linker concentration as a function of the sebum fraction in oil. The microemulsion phases (Types I, II, III, and IV) were obtained by visual observation. The effect of salinity (0.5 wt %, 1.5 wt%, and 3 wt % NaCl) and co-oil on the phase behavior was investigated. RESULTS AND DISCUSSION EACN OF CO-OIL AND OPTIMUM SALINITY Formulating microemulsions requires the right combination of variables that will pro vide an optimum middle-phase microemulsion. Salager et al. (42) proposed a semiem pirical equation that relates the different formulation variables: ln(S*) = k(EACN) + /(A) - er+ arD..T (1) where S* is the optimum salinity, or electrolyte concentration k is a constant, normally between 0.1 to 0.17 and EACN is the equivalent alkane carbon number for nonlinear hydrocarbon (e.g., triglycerides). For linear alkane hydrocarbons, the alkane carbon number (ACN) is applied. The EACN is estimated based on the optimum salinity obtained in our formulation studies the higher the optimum salinity required, the higher the hydrophobicity or EACN of the oil. The effect of alcohol or additives is noted by f(A)! er is a function of the type of the surfactant, a is a constant, Tis the temperature of the system, and D..T is the difference in temperature between the temperature of the system and an arbitrary reference temperature. However, in this study, alcohol is not included and the temperature of the system is constant. Acosta et al. (12,39) determined the EACN of squalene and isopropyl myristate (IPM) as shown in Table II (24 for squalene and 13 for IPM). The EACN for squalane is expected to be close to the value for squalene (-24). Table III shows the optimum salinity of oil mixtures (co-oil and sebum mixtures). The optimum salinity of pure isopropyl myristate is 3.5% NaCl, whereas the optimum salinity is lower when the amount of sebum oil is increased (e.g., the optimum salinity for the oil mixture of 20% vol. IPM and 80% vol. sebum is less than 0.5%). This suggests that IPM has a higher EACN or is more hydrophobic than sebum oil. The optimum salinity of pure ethyl laurate (EL) is 1-1.5% NaCl, which is closer to the optimum salinity of the 20% vol. EL and 80% vol. sebum mixture, indicating that EL has an EACN closer to sebum oil than IPM does (EACNsebum EACNEL EACN1pM). This is a very important finding in formulating cleansing products because the amount of sebum in human skin can be different depending on skin types. The ideal objective is to be able to formulate a
316 JOURNAL OF COSMETIC SCIENCE Table III Optimum Salinities for Oil Mixtures Isopropyl myristate (IPM)-sebum mixtures Ethyl laurate (EL)-sebum mixtures % 1PM % Sebum S* (%) % EL % Sebum S* (%) 20 80 0.5 20 80 0.5 40 60 0.5 40 60 0.5 60 40 0.8 60 40 0.5 80 20 1.5 80 20 0.5-0.7 100 0 3.5 100 0 1-1.5 product that is robust over a wide range of sebum oil secretion rates. One of the strategies to avoid this problem is finding a co-oil that has an EACN similar to that of the sebum oil, resulting in a similar optimum salinity. Therefore, based on this study, ethyl laurate is the best co-oil among the co-oils studied here, in terms of the robustness of the phase behavior. EFFECT OF SEBUM FRACTION IN OIL AND SURFACTANT CONCENTRATIONS ON PHASE DIAGRAM ([NaCl} = 0.5% wt) Microemulsion phase transition. The fish diagram for the system with squalene as co-oil at 0.5% NaCl is shown in Figure 3 the fish "tail" is observed in the high concentration region, whereas the fish "body" appears in the lower concentration region (Winsor Type III). The surfactant system was previously described in the Experimental Procedures section. The minimum surfactant concentration studied here was 14.19 wt%. At lower surfactant concentrations, very slow phase separation kinetics made it difficult to map out the remainder of the three-phase region. The surfactant concentration and the sebum fraction of oil at which the body and tail of the fish meet are denoted by "C" and "F," respectively. The concentration C for this system, approximately, is 25 wt% surfactant concentration at the fraction F of 0.42, as summarized in Table IV. When the fraction of sebum in oil is zero (i.e., 100% squalene), Type I microemulsion forms at the surfactant concentration studied. Without the co-oil (when sebum fraction is equal to one), a Winsor Type I microemulsion forms only at very high surfactant concentrations, but no microemulsion forms at lower surfactant concentrations. When the sebum frac tion in the oil increases, a Winsor Type I-III-II transition is observed at a low surfactant concentration regime up to 25% total surfactant concentration. A Winsor Type I-IV-I transition appears at high surfactant concentrations, although at intermediate surfactant concentrations, a Winsor I-IV-II transition occurs with an increase in sebum fraction in the oil. As shown in Table I, almost one-third of the sebum is fatty acids, which contribute to the high hydrophilicity of the sebum oil as compared to triglycerides. Squalene is a long-chain hydrocarbon oil that is present in the artificial sebum. Using squalene as co-oil in the microemulsion-based formulation can provide an efficient environment for the complicated comb-like structured triglycerides, enhancing the solubilization ability for artificial sebum. The co-oil can tune the spontaneous curvature of the surfactant monolayer, can help break up the structured triglyceride liquid phase (or liquid tri glyceride that is rigid, making it hard for surfactants to penetrate), and can increase the flexibility of the surfactant film, similar to the effect of adding a short-chain alcohol (5,7). Both effects are probably due to an increased interaction of squalene with the hydrocarbon region of the surfactant system, leading to a high degree of interaction
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)