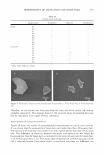
MICROEMULSIONS OF TRIGLYCERIDE-BASED OILS 323 50 � 40 0 30 ::I � 20 10 0 0 0.2 0.4 0.6 0.8 1 Sebum fraction in oil Figure 8. Fish diagram with squalane (solid line) and ethyl laurate (EL) (dashed line) at 1.5% NaCl as a function of surfactant concentration and sebum fraction in oil (a value of O is 100% co-oil and 1 is 100% sebum oil). Surfactant/linkers studied here are AOT (4%), hexylglucoside (5.06%), and sorbitan monooleate (5 .13% ). The concentration ratio is kept constant as the total surfactant/linker concentration is varied (25 ° C). Refer to Figure 4 for the phase behavior at 1.5% NaCl. vature with pure squalene at 0.5% NaCl, as shown in Figure 3. The addition of 1.5% NaCl makes the system become more hydrophobic and helps decrease the curvature of the surfactant membrane. Using co-oil that has a lower EACN, such as ethyl laurate, further decreases the curvature. As seen in Figure 8, a Type III microemulsion is observed in the absence of the sebum oil (the sebum fraction is zero). As the hydro philicity of the oil increases by increasing the sebum fraction in the mixed oil, the interaction between surfactant and oil is enhanced. This leads to a negative curvature, and the Type II microemulsion is formed. For the system with squalane, which is a more hydrophobic oil compared to ethyl laurate, a higher fraction of sebum oil is required to obtain the same curvature obtained from the system with ethyl laurate. CONCLUSIONS We have demonstrated that biocompatible and cosmetically friendly surfactants/linkers can be used to form single-phase triglyceride microemulsions (Winsor Type IV) at room temperature and low salt concentrations. The fish diagram observed in this study follows the classical patterns of phase behavior established for nonionic oligoethylene glycol based surfactants. We have also found that using co-oil helps the solubilization of triglycerides at lower surfactant concentrations and reduces the amount of surfactant necessary to form a single-phase microemulsion, which is desirable in cosmetic appli-
324 JOURNAL OF COSMETIC SCIENCE cations. For high-salinity systems, the amount of co-oil required to form a single-phase microemulsion increases as well as the surfactant concentration. This is an important consideration in the formulation of optimal skin cleansers, as the concentration of salt and the amount of sebum oil produced on human skin, as well as skin temperature, can be highly variable. Thus, a thorough understanding of phase behavior can be used to control the microstructure that forms in situ during skin cleansing. ACKNOWLEDGMENTS This research was supported by Mary Kay, Inc. REFERENCES (1) M. Bourrel and R. Schecter, Microemulsions and Related Systems: Formulation, Sovency, and Physical Properties, Surfactant Science Series 30 (Marcel Dekker, New York, 1988), pp. 1-483. (2) N. Garti, V. Clement, M. Fanum, and M. E. Leser, Some characteristics of sugar ester nonionic microemulsions in food applications, J. Agric. Food Chem., 48, 3945-3956 (2000). (3) P.A. Aiken and S. E. Friberg, "Microemulsions in Cosmetics," in Handbook of Microemulsion Science and Technology, P. Kumar, and K. L. Mittel, Eds. (Marcel Dekker, New York, 1999), pp. 773-787. (4) J. Daicic, U. Olsson, H. Wennerstrom, Phase equilibria of balanced microemulsions, Langmuir, 11, 2451-2458 (1995). (5) C. Von Corswant, S. Engstrom, and 0. Soderman, Microemulsions based on soybean phosphatidyl choline and triglycerides: Phase behavior and microstructure, Langmuir, 13, 5061-5070 (1997). (6) C. Von Corswant, P. Thoren, and S. Engstrom, Triglyceride-based microemulsion for intravenous administration of sparingly soluble substances,]. Pharm. Sci., 87, 200-208 (1998). (7) C. Von Corswant and 0. Soderman, Effect of adding isopropyl myristate to microemulsions based on soybean phosphatidylcholine and triglycerides, Langmuir, 14, 3506-3511 (1998). (8) C. Von Corswant, C. Olsson, and 0. Soderman, Microemulsions based on soybean phosphatidylcholine and isopropyl myristate-Effect of addition of hydrophilic surfactants, Langmuir, 14, 6864-6870, (1998). (9) B. Jakobs, T. Sottmann, and R. Strey, Amphiphilic block copolymers as efficiency boosters for microemulsions., Langmuir, 15, 6707-6711 (1999). (10) H. Kunieda and M. Yamagata, Mixing of nonionic surfactants at water-oil interfaces in microemul sions, Langmuir, 9, 3345-3351 (1993). (11) L. Huang and A. Lips, Microemulsification of triglyceride sebum and the role of interfacial structure on bicontinuous phase behavior, Langmuir, 20, 3559-3563 (2004). (12) E. J. Acosta, T. Nguyen, A. Witthayapanyanon, J. H. Harwell, and D. A. Sabatini, Linker-based bio-compatible microemulsion, Eviron. Sci. Technol., 39, 1275-1282 (2005). (13) P.A. Winsor, Solvent Properties of Amphiphilic Compounds (Butterworth, London, 1954), pp. 1-207. (14) R. Strey, Microemulsion microstructure and interfacial curvature, Colloid Polym. Sci., 272, 1005-1019 (1994). (15) J. A. Sillas and E.W. J. Kaler, The phase behavior and microstructure of efficient cationic-nonionic microemulsions,J. Colloid Interface Sci., 243, 248-254 (2001). (16) J. Alander and T. Warnheim, Model microemulsions containing vegetable oils. Part 1: Nonionic surfactant systems,]. Am. Oil Chem. Soc., 66, 1656-1660 (1989). (17) J. Alander and T. Warnheim, Model microemulsions containing vegetable oils. Part 2. Ionic surfactant systems,]. Am. Oil. Chem. Soc., 66, 1661-1665 (1989). (18) R. F. Joubran, N. Parris, D. P. Lu, and S. Trevino, Synergetic effect of sucrose and ethanol on formation of triglyceride microemulsions,J. Dispers. Sci. Technol., 15, 687-704 (1994). (19) N. Parris, R. F. Joubran, and D. P. Lu, Triglyceride microemulsions: Effect of nonionic surfactants and the nature of the oil,]. Agric. Food. Chem., 42, 1295-1299 (1994). (20) P. Skagerlind and K. Holmber, Effect of the surfactant on enzymic hydrolysis of palm oil in micro emulsion, J. Dispers. Sci. Technol., 15, 317-332 (1994).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)