ADHERENCE OF VEGETABLE BATH OILS 615 Oil Preparations As stated, the three bath oils used in this study were mineral oil, and soybean and cottonseed oils. Two different surfactants, PEG-400 (poly- ethylene glycol dilaurate) and Igepal RC-520 [nonylphenoxy (ethyl- eneoxy) ethanol], were added to the oils at 10 and 8% levels, respec- tively, to produce six preparations. The oil and surfactant preparations (mixtures) were subsequently added to water to yield a 0.02% solution (emulsion). Other additives normally present in various commercial bath oils were not used in this study. Incubation Procedures The keratin, 200 mg, was added to a 50-ml centrifuge tube. Bath oil preparations were added from a tared microliter syringe to the inside wall of the centrifuge tube, with care not to apply the oil directly onto the keratin. Exactly 40 ml of water was then added to each tube, the tubes were sealed with Teflon screw caps, and the emulsions were formed im- mediately using a vortex tube mixer. The emulsions were formed in situ rather than in a master batch, since experiments using a dye (Evans blue) showed that there was some layering of oil even in water-oil sys- tems that had been even more vigorously (sonification) dispersed. The approximate analysis of the water used in this study was as follows (mg/1000 g): Total dissolved solids, 235.0 total hardness (cal- cium carbonate), 115.0 CI-, 7.0 SO4 =, 16.0 NO:s-, 1.5 F-, 0.1 HCO:•-, 129.0 CO:s =, 0.0 Ca++, 30.0 Mg++, 9.0 Fe +++, 0.02 Na+, 4.0. As soon as the emulsions were formed, the tubes were placed in a Dubnoff-constant temperature (37øC) water bath and shaken for 30 minutes. After incubation, the tubes were centrifuged for ten minutes at 3000 rpm, the water-bath oil was decanted, and the extraction or washing procedures were begun. Ex traction Procedures After the water-bath oil was decanted from the keratin, a sequential extraction of the keratin was begun. The keratin was washed twice, a 10- and 5-ml wash, with each of the following solvents: distilled water, hexane, diethyl ether, and 2:1 chloroform:methanol. Each time a sol- vent was added to the keratin, it was shaken vigorously using the vortex test tube mixer and then centrifuged. With the chloroform:methanol extraction, however, it was necessary to filter the solvent away from the keratin, since the resultant dehydrated-defatted protein floats in this ex-
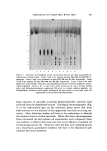
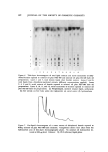
616 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS tracting solvent. The water washings were extracted with 5 ml of hexane. All organic extracts were evaporated to dryness under nitrogen. Naturally occurring lipids are extracted from tissue depending upon a number of factors (5). These include: (a) the solubility of the lipid or lipid-like material in the extracting solvent (b) the solubility of tissue water in the extracting solvent (namely, whether the extracting solvent can penetrate both surface and bound water of the tissue sufficiently to remove or dissolve the water surrounding the protein or other cellular material and thereby dissolve and extract the lipid material) and (c) the ability of the extracting solvent to remove or disassociate chemically bound lipid material. The sequential extraction used in this study is therefore based upon the ability of the extracting solvents water, hexane, diethyl ether, and chloroform-methanol (2:1) to remove the bath oils in a manner similar to that of naturally occurring tissue lipids, namely, the degree (binding capacity) to which these bath oils have bound to keratin. ./lnaly tical Procedures The analytical procedures were designed so that they were capable of accurately measuring the extracted oils in the microgram range. At the same time, it was necessary to differentiate between the naturally oc- curring triglycerides and hydrocarbons in the keratin and those added as bath oil. The desired analysis was accomplished with the aid of gas-liquid chro- •natogTaphy (GLG) and thin-layer chromatography (TLG). The GLG instrument used was an F&M Model 400 gas-chromatograph equipped with an H2-fiame detector with a lower range of detection, under ideal conditions, of 1 X 10 -9 g (nanogram). The vegetable oils were measured by first converting their fatty acid esters to methyl esters. Typical chromatogTams showing the fatty acid ester composition of cottonseed oil and soybean oil are shown in Figs. 1 and 2, respectively. The column used for this type of analysis was a 6-ft X 1/8-in. glass column packed with 80-90 mesh Anachrom ABS* coated with 10% ethylene glycol succinate. Methylation of the vegetable oils was accomplished by simply allowing milligram amounts of the oils to react at room temperature (with occasional shaking) for 15 minutes with an anhydrous 0.5N NaOH methanol solution (1 cello mg oil). This method will methylate other lipid esters but will not quantitatively * Analabs, Inc., Hamden, Conn.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)