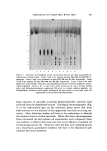
CHEMICAL DIFFERENTIATION IN EPIDERMIS 587 Skin 0.24M NH•C1, pH 9.5, 0øC, 15' Epidermis Homogenize, 8M Urea-0.2M Tris, pH 8.5 Urea Extract Dialyze-dilute NH•OH Lyophilize Dermis Urea Insoluble Dried Residue 0.1N HCIO•, 24øC, 30' Centrifuge 0.1N HC104 Soluble pH 4.5 pH 4.5 Precipitate 2M Na2COa Carbonate Extract Sephadex Chromatography pH 4.5 Supernatant Insoluble Residue 0.1N HC104 Insoluble 0.5N HC10•, 80øC, 30' 0.5N HCIO• Soluble Sephadex Chromatography "Glycine-Rich" Protein "Itistidine-Rich" Protein (GP) (HP) 0.5N HCIO4 Insoluble (Final Residue) Figure 2. Procedure used to isolate the "histidinc-rich" and "glycine-rich" proteins from a urea extract of the newborn rat epidermis. Modified from ref. (6) homogenization of the isolated epidermis in 8M urea, pH 8.5, is dialyzed against dilute ammonia and the residual (nondialyzable) protein solu- tion is lyophilyzed. After further extensive drying in vacuo, the protein is treated with 0.1N HC104 (PCA) at 24øC and the solubilized protein is removed from the insoluble residue by centrifugation. HP is isolated from this "0.1N PCA-soluble" fraction by isoelectric precipitation and chromato•aphy on Sephadex G-50 and G-100. GP arises from exposure of the "0.1N PCA-insoluble" fraction to 0.5N PCA at 80øC and removal of the solubilized protein by centrifugation leaving the "hot PCA- insoluble" residue. The GP is recovered from the "hot PCA-soluble" fraction by chromatography on Sephadex G-50. Table II is a compilation of data on amino acid composition of the HP, GP, "urea-extractable" fraction, and the "hot PCA-insoluble" frac- tion from the epidermis of the newborn rat. Also included in this table is the composition of HP isolated from human epidermis (11). The most striking characteristics of the HP and GP are the levels of histidine and glycine, respectively, the absence of sulfur-containing residues in both, and the absence of apolar amino acids other than glycine and alanine in the HP. Since the GP, when analyzed for amino acid composition, was not as well purified as was the HP, it is entirely possible
588 .JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table II Amino Acid Composition • of Various Fractions of Protein from the Newborn Rat Epidermis and from Human Epidermis s Rat Urea- PCA- extract HP GP insoluble Human HP Glutamic acid 14.8 14 Serine 11.2 11 Glycine 15.8 15 Arginine 6.8 9 Alanine 7.6 11 Tyrosine 2.7 9 Histidine 2.5 6 Threonine 4.8 6 Aspartic acid 8.3 5 Lysine 4.6 1 Leucine 6.2 0 Isoleucine 3.1 0 Phenylalanine 2.6 0 Proline ... 0 Valine 3.9 0 •/• cystine ... 0 Methionine ... 0 (NH3) ... (6 a Residues/100 residues. 1 11.6 12.4 5 19.2 7.2 3 33.8 15.6 0 6.9 4.3 8 5.9 7.3 4 2.7 3.7 9 3.2 0.8 8 3.8 5.4 6 3.9 8.6 0 2.1 3.7 1.0 8.1 1.0 4.3 1.7 2.7 0 1.4 1.7 6.3 0 0.5 0 1.4 .8) ... (11.0) 12 0 60 16 8 10 0 101 43 83 10.7 15.4 1.3 1.6 1.2 0.6 , . . 1.8 0 0 ß . . b Sourcesofdata: rat urea-extractandGP (6) rat HPandPCA-insoluble(12) human HP (11). that the only apolar residues in the GP may also be glycine and alanine. Further purification of the GP, whose molecular weight is about 5000, will be necessary to clarify this point. Most recent data (12) indicate that the HP constitutes about 2.5% of the "urea-extractable" protein and contains about 35% of the labeled amino acid in the epidermis one hour after the intraperitoneal adminis- tration of histidine-all to the newborn rat. The GP has about 10% more of the protein and about 14% more of the '•H. ChromatogTaphy on Sephadex indicates that the HP is part of a much larger protein prior to treatment with 0.1N PCA. Whereas passage of the "urea-extractable" protein, after dialysis, through a column of Sephadex G-200, indicates all the radioactive protein to have a molecular or "complex" weight in excess of 400,000 (all the protein being ex- cluded), the HP on Sephadex G-100 assumes the position of molecular weight about 30,000, assuming a globular protein. HP is retarded on G-100 but excluded on G-50 (12).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)