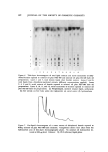
CHEMICAL DIFFERENTIATION IN EPIDERMIS 591 "urea-extractable" protein (after dialysis) is soluble in 0.1N PCA and that nearly 80% of the solubilized radioactive protein is precipitable at pH 4.5 (19). Although these observations do not unequivocally say that the HP is converted to keratohyalin, they strongly support this view. Furthermore, a small amount of HP has been isolated froin keratohyalin which was obtained from a "granular-cornified" cell preparation of the newborn rat epidermis by a recently described method (20) for extracting keratohyalin from bovine hoof tissue. Additional support for the postu- lated relationship between the HP and keratohyalin is the finding that in psoriasis, the involved human epidermis, which has no keratohyalin, does not form the HP whereas normally keratohyalin is present and the HP is made (11). Since the HP constitutes the major fraction of radioactive "urea- extractable" protein over the period of time during which incorporated histidine-:•H moves from outside to inside the keratohyalin in the granular cells of the newborn rat skin, it seems reasonable to consider that when the HP becomes part of keratohyalin only a physical change occurs, i.e., the molecule is not chemically altered. NATURE OF THE BOND IN EPIDERMAL PROTEIN CLEAVED BY 0.1N PERCHLORIC ACID The formation of soluble protein when epidermal "urea-extractable" protein is exposed to 0.1N PCA at room temperature suggests that epidermal protein contains a linkage which is not usually found in pro- tein. Since the HP does not contain detectable cysteine residues, PCA cannot mediate this cleavage by oxidation of disulfide linkages. In fact, it appears that 0.1N HC1 can substitute for PCA (21). That the frag- mentation is not indiscriminate is indicated by the size of the HP, the finding of single N- and C-terminal amino acid residues in the HP, the unusual amino acid composition of this protein, and the similarity of the HP obtained from the human to that of the rat. The fact that the HP always accounts for a large percentage of the incorporated histi- dine-:*H in the epidermis lends support to this conclusion. Figure 3 diagrams a structure which appears to coincide with all presently available data on the HP and its original inclusion in the "urea- extractable" protein. It is proposed that the HP is linked to a much larger protein through the copper ion and urocanic acid. Treatment with PCA hydrolyzes the peptide linkage through which urocanic acid is bound to what then becomes the "PGA-insoluble" protein. In this
592 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (urocanic acid) 0 ,, - CH = CH - C --• NH - CH ,, : CH^OH (ar91nine) ' COLD• INSOLUBLE PROTEIN (•H2)3 ,[ ', +H3N - CH ' •- NH - PROTEIN - NH - CH - R ,, I I •ISTIDINE 0 C - O- PROTEIN (lysine) i, I o I (arginine) j •"igure 3. Hypothetical structure for the "histidine-rich" protein in its "native" structural environment process previously absent N-terminal amino acids appear, e.g., glycine, serine, threonine, etc. PCA also causes the release of urocanic acid and copper ion which become dialyzable. The manner in which the copper ion binds the HP is not known and is not shown. Copper ion is assumed to bind to the imidazole group of urocanic acid in a way which prevents the titration of this •oToup until the protein is exposed to PCA. The proposed structure is consistent with the observation that the N-terminal residue of the "urea-extractable" protein and the HP is lysine with its •-amino group blocked in some manner. The structure also accounts for the finding that the C-terminal residue of all fractions is arginine. The failure to find a new C-terminal amino acid to compli- ment the new N-terminal residues is explained by the proposal that urocanic acid, which would not have been observed by the technique used, provides the C-terminal. A major issue is whether the proposed "urocanyl" peptide linkage is more labile to dilute PCA than is the usual peptide linkage. This question is being studied. FORMATION OF "HISTIDINE-RICH" PROTEIN, A MODEL FOR STUDY OF CONTROL OF DIFFERENTIATION Availability of an in vitro system which synthesizes the HP would allow a study of the steps in the formation of this unique epidermal protein and the mechanism whereby its biosynthesis is "repressed" until the differentiating cell reaches the granular layer. Although it has been possible to demonstrate the synthesis of the HP in minces of new- born rat epidermis (22), no cell-free preparation of this tissue has been obtained which synthesizes the HP or any other protein. The presence
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)