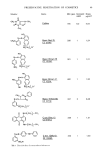
80 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS PENTANOL + OCTYL ALDEHYDE / WATER / SODIUM DODECYL SULFATE + OCTYL ALDEHYDE Figure 6. Introducing octyl aidehyde gave a trend similar to the one found for methyl stearate. Octyl aldehyde/(Octyl aidehyde + pentanol) Octyl aldehyde/(Octyl aidehyde + sodium dodecyl sulfate) -- ß -- 0.25 of the water and oil continuous areas at as small a fraction of ethyl acetate as 0.! is a result of the destabilizing action of the ethyl acetate on the lameliar liquid crystalline phase that is found between the W/O and O/W areas in the basic system (12). A similar action has been found for short chain alcohols. In the water/soap/alcohol systems (13) the large liquid crystalline region separating the unusual and inverse miceliar areas has been found for alcohols with a chain length of five and higher. For butanol and shorter alcohols the destabilizing action on the liquid crystal gave a continuous solubility area from pure butanol to water without an interruption by a liquid crystalline phase. Esters with intermediate chain lengths showed identical trends, but to a more modest degree. For butyl acetate, the short hydrocarbon chains still meant a reduced destabilizing effect, the the coalescence between W/O and O/W areas was now found first at higher fractions of the ester. The opposite effect, a stabilization of the more
MICROEMULSIONS WITH ESTERS 81 ordered phase, was found for methyl stearate. Methyl stearate actually gave smaller W/O microemulsion areas than a hydrocarbon would (11). We attribute this reduction to a stabilization of lameliar liquid crystal toward higher fractions of ester. A second factor that cannot be ruled out, is the possibility of crystallization of the methyl sterate. The solubility regions of the butoxyethanol are an interesting extension of the theme of relative stability of the liquid crystal W/O micro-emulsion structures. It appears that the enhanced polarity leads to enhanced destabilizing action on the liqud crystalline phase. SUMMARY The results showed microemulsions containing esters and other polar substances to behave in a predictable manner. The deviations from the characteristics of hydrocarbon microemulsions were explained as arising from the presence of the polar group that led to the enhanced concentration of the compound at the oil/water interface. For short chain compounds this mechanism led to destabilization of more ordered phases, resulting in larger microemulsion areas, while the long chain compounds gave the opposite consequence. REFERENCES (1) D. Bowden andJ. Holmstine, U.S. Patent 2,045,455 (1936). (2) V. R. Kokatnur, U.S. Patent 2,111,100 (1935). (3) T. P. Hoar and J. H. Schulman, Transparent water-in-oil dispersions: the olepathic hydro-micelle, Nature, 152, 102 (1943). (4) H. L. Rosano, Microemulsions, J. Soc. Cosmet. Chem., 25,609 (1974). (5) L. M. Prince, Microemulsions versus micelies, J. Colloid Interface $ci., 52, 182 (1975). (6) C. A. Miller and L. E. Scriven, Interfacial instability due to electrical forces in double layers. I. General considerations, Ibid., 33, 361 (1970). (7) E. Ruckenstein, Thermodynamics of microemulsification with ionic surfactants, J. Dispersion Sci. Technol., 2, 1 (1981). (8) L. M. Prince, A theory of aqueous emulsion. I. Negative interfacial tension at the oil/water interface, J. Colloid Interface Sci., 23, 165 (1967). (9) D. O. Shah and R. S. Schechter, Eds., Improved Oil Recovery by Surfactant and Polymer Flooding (Academic Press, New York, 1977). (10) D. O. Shah, Ed., Surface Phenomena in Enhanced Recovery (Plenum Press, New York, 1981). (11) S. Friberg and I. Burasczewska, Microemulsions in the water-potassium oleate-benzene system, Progr. Colloid Polym. Sci., 63, 1 (1978). (12) E. Sjoblom and S. Friberg, Light-scattering and electron microscopy determinations of association structures in W/O microemulsions,J. Colloid lnteface Sci., 67, 16 (1978). (13) P. Ekwall, Composition, properties, and structures of liquid crystalline phases in systems of amphiphilic compounds, Advances in Liquid Crystals, Vol. 1, G. H. Brown, Ed. (Academic Press, New York, 1975).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)