ALLERGENS OF LANOLIN 121 400 ml of methanol, and 2.6 g of sodium methylate were added to the electrolytic cell. Electrolysis was done at 2 amp. for 32 hr. The temperature of the reaction mixture was kept below 50øC by cooling with ice. The reaction was worked up as follows. Methanol was distilled off under vacuum, and 1 1. of ether was added to the residue. The ethereal layer was washed three times with 10% sodium carbonate solution and then with 10% sodium chloride solution until neutral. The organic layer was dried and the solvent distilled off under vacuum to afford 96.6 g of an oily material. The crude product was hydrolyzed and then purified by vacuum distillation. (XI) b. p. 118-120 ø (9.5 ram), yield 36.6%, MS (methyl ester) m/e 158 (M+), IR (neat) 1700 cm -•, GLC 98.2%. (XII) b. p. 135-136 ø (9 mm), yield 30.7%, MS (methyl ester) m/e 172 (M+), IR (neat) 1700 cm -•, GLC 98.5%. (XlII) b. p. 178-179 ø (4 mm), m.p. 61-62 ø, yield 22.7%, MS (methyl ester) m/e 270 (M+), IR (neat) 1700 cm -•, GLC 98.7%. 6 o (XIV) b. p. 175-176.5 ø (5 mm), m.p. 3 -37 , yield 28.5%, MS (methyl ester) m/e 284 (M+), IR (neat) 1700 cm -•, GLC 99.0%. ot-Hydroxy fatty acids (XVIII, XIX and XX). c•-Brominations of palmitic acids, XIII and XIV were done by the procedure of Schwenk et al. (10). Then, the products were hydrolyzed to afford the corresponding c•-hydroxy fatty acids, XVIII, XIX and XX. Repeated recrystallization of the product from n-hexane afforded the following colorless crystals: (XVIII) m.p. 69.5-70.0 ø, Anal. Calcd. for C•6H3203, C 70.5%, H 11.8%, Found C 70.3%, H 11.8%, IR (KBr) 3500, 1730, 1110, 900 cm -•. (XIX) m.p. 46.0-47.0 ø, Anal. Calcd. for C17H3403, C 71.3%, H 12.0%, Found C 71.2%, H 11.8%, IR (KBr) 3500, 1730, 1110, 900 cm -•. (XX) m.p. 86.0-86.7 ø, Anal. Calcd. for C16H3203, C 70.5%, H 11.8%, Found C 70.3%, H 12.0%, IR (KBr) 3500, 1730, 1110, 900 cm -•. Alkane-l,2-diols (XXL XXII and XXIII). Methyl esters of XVIII, XIX and XX were reduced with lithium aluminum hydride in ether to give XXI, XXII and XXIII, respectively. These products were purified by repeated recrystalization from acetone. (XXI) m.p. 50.2-50.7 ø, Anal. Calcd. for C16H3402, C 74.4%, H 13.3%, Found C 74.0%, H 13.3%, IR (KBr) 3250, 1080 cm -•. (XXII) m.p. 36.5-37.5 ø, Anal. Calcd. for C•7H3602, C 74.9%, H 13.3%, Found C 74.8%, H 13.4%, IR (KBr) 3250, 1080 cm -• (XXIII) m.p. 75.0-76.0 ø, Anal. Calcd. for C16H3402, C 74.4%, H 13.3%, Found C 74.1%, H 13.3%, IR (KBr) 3250, 1080 cm -•. 3-Methyl-11-dodecenoic acid (XXIV). Magnesium (3.89 g) and small amounts of iodine were added to 27 g of V dissolved in 50 ml of anhydrous ether. The suspension was heated with vigorous stirring until the reaction started, and stirred with gentle refluxing for an additional 2 hrs. After cooling to room temperature, the solution was added to anhydrous ether containing excess dry ice, and stirred for 3 hrs. The resulting solution was acidified with 5% aqueous sulfuric
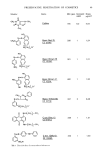
122 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS acid. Then 200 ml of ether were added and separated. The ethereal layer was washed with 10% sodium chloride solution until neutral and dried. The ether was vacuum distilled to afford 25.9 g of oil () ield 91.5%, GLC 91.4%) which was purified by vacuum distillation. (XXIV) b. p. 137.5-139.5 ø (0.5 ram), MS (methyl ester) m/e 226 (M +), IR (neat) 1710, 1640, 1300, 990, 910 cm -• GLC 99.2%. , Hydroboration (XXV, XXVI, and XXVII). w-Hydroxy fatty acids, XXV-XXVII, were prepared by the hydroboration technique of Brown et al. (11). The crude products were purified by repeated recrystallization from benzene yielding colorless crystals. (XXV) m.p. 65.5-66.0 ø, Anal. Calcd. for CllH2203, C 65.3%, H 11.0%, Found C 65.2%, H 11.1%, IR (KBr) 3450, 1710, 1200, 1180, 1050 cm-k (XXVI) m.p. 41.0-42.0 ø, Anal. Calcd. for C12H2403, C 66.6%, H 11.2%, Found C 66.5%, H 11.3%, IR (KBr) 3200, 1710, 1050, 940 cm-k (XXVII) m.p. 56.5-57.0 ø, Anal. Calcd. for C•3H2603, C 67.8%, H 11.4%, Found C 67.7%, H 11.4%, IR (KBr) 3350, 1710, 1300, 1050 cm -• 2-Methylundecane-l,ll-diol (XXIX) and 3-methyldodecane-l,12-diol (XXX). Methyl esters of XXVI and XXVII were reduced with lithium aluminum hydride in ether to afford XXIX and XXX, respectively. These were purified by repeated recrystalization from acetone to give colorless crystals. (XXIX) m.p. 28.7-29.2 ø, Anal. Calcd. for C•2H2602, C 71.2%, H 13.0%, Found C 71.5%, H 13.2%, IR (neat) 3300, 1050 cm -•. (XXX) Anal. Calcd. for C•3H2702, C 72.2%, H 13.0%, Found C 72.0%, H 12.9%, IR (neat) --1 3300, 1050 cm RESULTS AND DISCUSSION PATCH TESTS WITH THE SYNTHETIC DIOLS Alkane-c•,/•-diols and alkane-c•,o•-diols in hydrogenated lanolin are mixtures of homologs of C,4-C25 (a main component n-C•6) and C28-C33 (a main component n-C30), respectively. In addition, they have three isomeric series, so called normal-, iso- and anteiso-isomers. The iso-series includes only even carbon number homologs and the anteiso-series only odd ones (12). Our previous work demonstrated that these diols possessed allergenicity as a mixture of homologs and isomers. It was our objective, therefore, to determine which isomeric series or homologs within the mixture possess allergenicity. Patch tests with the synthetic diols were carried out as 10% solutions in olive oil. The results are shown in Table I. In the case of alkane-c•,o•-diols, only the iso-isomer of the C• homolog elicited allergic reactions. However, the allergenicity was appreciably lower than that of the isolated diols from hydrogenareal lanolin. Although the normal isomer of alkane-c•,/•-diol was not allergenic, the iso- and anteiso-isomers except anteiso-C•3 definitively showed allergenicity. It is presumably possible to note that the allergic potential varies with their total carbon number. In
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)