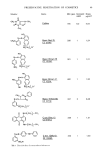
ALLERGENS OF LANOLIN 117 investigations on the allergy have been done since 1929 (2). However, allergens of lanolin have been unidentified because of the complicated chemical composition of lanolin. Only Truter et al. (3) isolated 7,11-dioxo-lanosta-8-en-3-ol from lanolin as a weakly allergenic compound. Our previous investigation (4) demonstrated that the major allergenic constituents of hydrogenated lanolin, which possesses the highest allergenicity among various lanolin derivatives, were alkane-cr,/•-diols and alkane-cr,w-diols. However, these diols are a mixture of homologs and have three isomeric series, normal-, iso- and anteiso-isomers. It is noteworthy from the biogenetic point of view that the iso-series has an even total number of carbon atoms whereas the anteiso-series has an odd number. Because human skin can have potentially different responses to these isomeric series, it is necessary to determine which isomeric series possess allergenicity. It seemed quite difficult to isolate each isomer in pure form from these diols because of the presence of homolog distributions. Therefore, our approach was to synthesize all types of isomers of these diols and to examine their allergenicity. Allergenicity was evaluated by human patch testing and guinea pig sensitization testing. EXPERIMENTAL APPARATUS AND TESTING PROCEDURES The apparatus was similar to that previously described (4). Human patch tests were carried out as previously described (4). Animal tests were done as follows. In intradermal sensitization experiments with guinea pigs, 0.5 ml of a test sample in Freund's complete adjuvant (FCA) was injected intradermaly. The injections were performed weekly for 4 weeks. In topical sensitiza- tion experiments, the applications were repeated daily on the same skin site for 10 days. Induction of allergic contact dermatitis was tested after 2 weeks on the flank of the guinea pig with the challenge sample by topical application. SYNTHESES Synthetic routes used to obtain various compounds are shown in Figures 1, 2, and 3. Most of these involve application of published procedures adapted to yield the compounds of interest. Where the adaptations involve significant departures from the published procedures the departures are described. Generally the procedures are referenced and the characteristics used to establish the synthesized materials' identities indicated. 2-Methyl-lO-undecenoic acid (I) and 2-ethyl-lO-undecenoic acid (H). Alkylations of 10-undecenoic acids were done by the procedure of Pfeifer et al. (5) The products after purification by vacuum distillation had the following characteristics: (I) b. p. 132-133 ø (0.4 ram), yield 81%, MS (methyl ester of I) m/e 212 (M+), •H-NMR (CDC13) 1.28 ppm (3H, d, J = 8Hz --CH,), GLC 99%. (II) b. p. 134-136 ø (0.3 mm), yield 78%, MS (methyl ester of//) 226 (M+), •H-NMR (CDC13) 0.92 ppm (3H, t, J = 8Hz --CH3) , GLC 99%.
118 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS CH2=CH(CH2)sCOOH 1. BuLi Diisopropylamine 2. MeI or EtBr 1. H + MeOH , 2. LiA1H4, ether R CH 2=CH(CH 2)7CHCOOH I R= Me R i CH 2=CH(CH2)7CHCH2OH ]lI R = Me •V•R= Et SOC12 , pyridine R ß CH2=CH(CH2)7CHCH2CI V R= Me VI R = Et LiA1H4, in THF R CH2=CH(CH2)7 CHCH 3 VII•R = Me VI•R = Et 1. H202, CH3COOH 2. OH R CH2-CiH(CH2)7CHCH 3 I OH OH IX R = Me X a = Et Figure 1. Scheme for the syntheses of alkane-c•,fi-diols. (iso-C•2 and anteiso-C•0. 1-Chloro-2-methyl- 10-undecene (V) and 1-chloro-2-ethyl- ! O-undecene (VI). Methyl esters of I and H were reduced with lithium aluminum hydride in ether giving alcohols, III and IV. Purification was by extraction. Yields of III and IV were 87 and 89%, respectively. Chlorinations of III and IV were done by the Brody et al. procedure (6). The products after purification by vacuum distillation had the following characteris- tics: (V) b. p. 106.5-108.5 ø (8.0 mm), yield 54.5%, MS m/e 202 (M+), IR (neat) 1640, 1290, 990, 720 cm -•, GLC 98.3%. (VI) b. p. 120-121.7 ø (4.5 mm), yield 42.6%, MS m/e 216 (M+), IR (neat) 1640, 1290, 990, 720 cm -•, GLC 99.5%. 1-Methyl-lO-undecene (VII) and 2-methyl-ll-dodecene (VIII). According to the procedures of Nystrom and Brown and Johnson et al. (7), V and VI were reduced in THF with lithium aluminum hydride at reflux temperature for 4 hr to
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)