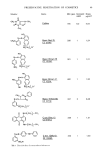
116 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (2) M. Chevreul, Note sur la nature du suint de mounton, C. R. Acad. Sci. Paris, 43, 130 (1856). (3) E. V. Truter, The activities of some water-in-oil emulsifying agents,J. Soc. Cosmet. Chem., 13, 173-187 (1962). (4) O. Kozuka, Contact dermatitis caused by lanolin, IgakuJournal, 1,171-177 (1976) (in Japanese). (5) G. Peter, F. Schrogl, and H. Franzwa, Experimentelle Untersuchungen uber die allergene Wirkung yon Wollwachsalkoholen, Hautarzt, 20, 450-455 (1969) E. W. Clark, Estimation of the general incidence of specific lanolin allergy,J. Soc. Cosmet. Ct•em., 26, 323-335 (1975), and the literatures cited in. (6) M. A. Ramirez and J.J. Eller, The patch test in contact dermatitis,J. Allergy, 1,489-495 (1929). (7) T. Mortensen, Allergy to lanolin, Contact Derm., 5, 137-139 (1979). (8) K. Wereide, Contact allergy to wool-fat ("lanolin"), Acta derm.-venereal., 45, 15-18 (1965). (9) T. Sugai andJ. Higashi, Hypersensitivity to hydrogenated lanolin, Contact Derm., 1,146-157 (1975). (10) T. Kozuka, T. Tangaki, T. Akimoto, M. Tashiro, K. Fujimoro, and S. Hashimoto, Cutaneous hypersensitivity to lanolin, Hif•, 18, 35-37 (1976) (in Japanese). (11) j. Everall and E. V. Truter, Cutaneous hypersensitivity to lanolin, investigation of one case, J. Invest. Derm., 22, 493-496 (1954) E. V. Truter, •rool •/ax (Cleaver-Hume Press Ltd., London, 1956), pp. 31-64. (12) Organic Reactions, Vol. 6, ed. R. Adams et al. (John Wiley & Sons, Inc., New York, 1957), pp. 469-509. (13) J. Diekmann, J. B. Thomson, and C. Djerassi, Mass spectrometry in structural and stereochemical problems. CLV: electron impact induced fragmentations and rearrangements of some trimethylsilyl ethers of aliphatic glycols and related compounds,J. Org. Chem., 33, 2271-2284 (1968). (14) D. T. Downing, Z. H. Kranz, and K. E. Murray, Studies in waxes. XIV: an investigation of the aliphatic constituents of hydrolysed wool wax by gas chromatography, AustralianJ. Chem., 13, 80-94 (1960). (15) S. Ito, K. Endo, S. Inoue, and T. Nozoe, 10(S)-Hydroxy-6(R), 9(S)-oxidohexadecanoic acid, a new acid in wool fat, Tetrahedron Lett., 4011-4014 (1971). (16) M. B. Sulzberger, T. Warshow, and F. Herrmann, Studies of skin-hypersensitivity to lanolin,J. Invest. Derre., 20, 33-43 (1953). (17) S. Takano, M. Yamanaka, K. Okamoto, and F. Saito, Allergens of lanolin II, allergenicity of synthetic alkane-c•,/3-diols and alkane-c•,w-diols,J. Soc. Cosmet. Chem., in press. PART II: ALLERGENICITY OF SYNTHETIC ALKANE-•x,•I-DIOLS AND ALKANE-•x,w-DIOLS Synopsis A previous study revealed that alkane-c•,/•-diols and alkane-c•,w-diols are major allergenic constituents of hydrogenareal lanolin. Aliphatic components of hydrogenated lanolin have not only straight alkyl chains (normal-series) but also branched alkyl chains, so-called iso- and anteiso-series. Because these isomeric series are considered to be different compounds in terms of human skin responses to them, syntheses and allergenicity of these three series of diols were investigated. It was found that the normal isomers of these diols showed no positive reactions. However, the iso- and anteiso-isomers caused allergic reactions in humans. In addition, sensitization of guinea pigs with synthetic iso-hexadecane-1,2-diol was performed. Epidermal challenges to the sensitized guinea pigs with commercial lanolin alcohol and hydrogenareal lanolin both gave positive results. These findings suggest that the branched compounds of these diols are major allergenic constituents of hydrogenated lanolin. INTRODUCTION Lanolin is a widely used ingredient in topical medicaments like ointments, cosmetic products, and many other household commodities. However, it has been known that lanolin and its derivatives cause allergic reactions with the same or greater frequency as allergens such as nickel, chromium, p-phenylenediamine and the like (1). A number of
116 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (2) M. Chevreul, Note sur la nature du suint de mounton, C. R. Acad. Sci. Paris, 43, 130 (1856). (3) E. V. Truter, The activities of some water-in-oil emulsifying agents,J. Soc. Cosmet. Chem., 13, 173-187 (1962). (4) O. Kozuka, Contact dermatitis caused by lanolin, IgakuJournal, 1,171-177 (1976) (in Japanese). (5) G. Peter, F. Schrogl, and H. Franzwa, Experimentelle Untersuchungen uber die allergene Wirkung yon Wollwachsalkoholen, Hautarzt, 20, 450-455 (1969) E. W. Clark, Estimation of the general incidence of specific lanolin allergy,J. Soc. Cosmet. Ct•em., 26, 323-335 (1975), and the literatures cited in. (6) M. A. Ramirez and J.J. Eller, The patch test in contact dermatitis,J. Allergy, 1,489-495 (1929). (7) T. Mortensen, Allergy to lanolin, Contact Derm., 5, 137-139 (1979). (8) K. Wereide, Contact allergy to wool-fat ("lanolin"), Acta derm.-venereal., 45, 15-18 (1965). (9) T. Sugai andJ. Higashi, Hypersensitivity to hydrogenated lanolin, Contact Derm., 1,146-157 (1975). (10) T. Kozuka, T. Tangaki, T. Akimoto, M. Tashiro, K. Fujimoro, and S. Hashimoto, Cutaneous hypersensitivity to lanolin, Hif•, 18, 35-37 (1976) (in Japanese). (11) j. Everall and E. V. Truter, Cutaneous hypersensitivity to lanolin, investigation of one case, J. Invest. Derm., 22, 493-496 (1954) E. V. Truter, •rool •/ax (Cleaver-Hume Press Ltd., London, 1956), pp. 31-64. (12) Organic Reactions, Vol. 6, ed. R. Adams et al. (John Wiley & Sons, Inc., New York, 1957), pp. 469-509. (13) J. Diekmann, J. B. Thomson, and C. Djerassi, Mass spectrometry in structural and stereochemical problems. CLV: electron impact induced fragmentations and rearrangements of some trimethylsilyl ethers of aliphatic glycols and related compounds,J. Org. Chem., 33, 2271-2284 (1968). (14) D. T. Downing, Z. H. Kranz, and K. E. Murray, Studies in waxes. XIV: an investigation of the aliphatic constituents of hydrolysed wool wax by gas chromatography, AustralianJ. Chem., 13, 80-94 (1960). (15) S. Ito, K. Endo, S. Inoue, and T. Nozoe, 10(S)-Hydroxy-6(R), 9(S)-oxidohexadecanoic acid, a new acid in wool fat, Tetrahedron Lett., 4011-4014 (1971). (16) M. B. Sulzberger, T. Warshow, and F. Herrmann, Studies of skin-hypersensitivity to lanolin,J. Invest. Derre., 20, 33-43 (1953). (17) S. Takano, M. Yamanaka, K. Okamoto, and F. Saito, Allergens of lanolin II, allergenicity of synthetic alkane-c•,/3-diols and alkane-c•,w-diols,J. Soc. Cosmet. Chem., in press. PART II: ALLERGENICITY OF SYNTHETIC ALKANE-•x,•I-DIOLS AND ALKANE-•x,w-DIOLS Synopsis A previous study revealed that alkane-c•,/•-diols and alkane-c•,w-diols are major allergenic constituents of hydrogenareal lanolin. Aliphatic components of hydrogenated lanolin have not only straight alkyl chains (normal-series) but also branched alkyl chains, so-called iso- and anteiso-series. Because these isomeric series are considered to be different compounds in terms of human skin responses to them, syntheses and allergenicity of these three series of diols were investigated. It was found that the normal isomers of these diols showed no positive reactions. However, the iso- and anteiso-isomers caused allergic reactions in humans. In addition, sensitization of guinea pigs with synthetic iso-hexadecane-1,2-diol was performed. Epidermal challenges to the sensitized guinea pigs with commercial lanolin alcohol and hydrogenareal lanolin both gave positive results. These findings suggest that the branched compounds of these diols are major allergenic constituents of hydrogenated lanolin. INTRODUCTION Lanolin is a widely used ingredient in topical medicaments like ointments, cosmetic products, and many other household commodities. However, it has been known that lanolin and its derivatives cause allergic reactions with the same or greater frequency as allergens such as nickel, chromium, p-phenylenediamine and the like (1). A number of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)