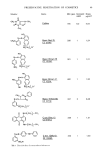
ALLERGENS OF LANOLIN 119 C H.•.. CH? CH3CH2x xCHCOOH CH 3 1.
CHCOOH Anodic synthesis, HOOC (CH 2) 4C00CH3 2. OH-, then H + R(CH2)4COOH X l R = g-Pr XlI R = $se-Bu 1. Anodic synthesis, HOOC (CH 2) 8COOCH 3 2. OH-, then H + R(CH2)12 COOH X•I R = i-Pr XIV R = s eo-Bu 1. SOC12, Br 2 2. EtOH Br I R (CH2)11CH COOCH2CH 3 XV R = i-Pr XVI a = 8ee-Su XV]-[ R = n-Pr 1. OH , dioxane, H20 2. H + OH I R(CH2)llCHCOOH X•rIlI R = i-Pr XlX R = see-Bu XX R = n-Pr 1. MeOH, H + OH i . R(CH2)11CHCH2OH 2. LiA1H 4, in ether XXI R = •-Pr XX]-[ R = sec-Bu xxm R = n-Pr Figure 2. Scheme for the syntheses of alkane-o•,fi/-diols. (n-C•6 , iso-C•6 and anteiso-C•7). afford VII and VIII, respectively. These products after purification by vacuum distillation had the following characteristics: (VII) b. p. 85-87 ø (9 mm), yield 40%, MS m/e 168 (M+), IR (neat) 1640, 990, 910 cm -•, GLC 99%. (VIII) b. p. 98-100 ø (9 mm), yield 58%, MS m/e 182 (M+), IR (neat) 1640, 990, 910 cm -•, GLC 99%. 10-Methylundecane-l,2-diol (IX) and 10-methyldodecane-l,2-diol (X). Oxidation of the terminal double bonds of VII and VIII was done by the procedures of
120 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS CH2=CH(CH2)7 CH ( R2 I R 1 = Me, R 2 XXIV R 1 = Me, R 2 = COOH = CH2COOH /R• HOCH 2CH2(CH2) 7 CH XR 2 XXV R 1 = H , R 2 = COOH XXVI R 1 = Me , R 2 = COOH XXV• R 1 = Me , R 2 = CH2COOH 1. bis-3-methyl-2-butylborane, in THF 2. H202, OH- 3. H + 1. H + MeOH 2. LiA1H4, in ether /R• HOCH2CH2(CH2) 7CH XR 2 XXIX • R 1 = Me , R 2 = CH20H XXX R 1 - Me , R 2 = CH2CH2OH Figure 3. Scheme for the syntheses of alkane-c•,c0-diols. (iso-C,2 and anteiso-C•3 ). Swern et al. (8). The crude products were purified by conversion to the diacetates, chromatography on silica gel, and hydrolysis of the chromatographically purified diacetates yielding IX and X: (IX) IR (neat) 3350, 1050 cm -•, •H-NMR (CDCI3) 0.86 (6H, d, j = 7Hz (CH3)2CH--), 1.1-1.6 (15H, br. s --CH2-), 3.28-3.67 ppm (3H, m --CH2(OH)CH(OH)--), Anal. Calcd. for C•H2602, C 71.2%, H 13.0%, Found C 71.4%, H 13.2%. (X) IR (neat) 3300, 1050 cm -•, •H-NMR (CDC13) 0.87 (6H, m --CH3), 1.1-1.6 (17H, br. s --CH2-), 3.28-3.67 ppm (3H, m -CH2(OH)CH(OH)-), Anal. Calcd. for C•3H•80•, C 72.2%, H 13.0%, Found C 72.0%, H 13.1%. ANODIC SYNTHESES The electrolytic cell was a glass 2 1 cylinder fitted with a cooling coil, a reflux condenser, and two platinum electrodes (4 x 2.5 cm). The electrolytic solvent was methanol containing sodium methoxide (1-2% based on the free acid content). Electrolysis was done at 2 amp. and discontinued when the solution became slightly alkaline (pH 7.5 or more). Mono methyl esters of adipic acid and sebacic acid were prepared according to Organic Syntheses (9). The following examplifies the procedure showing the synthesis of XI. 2-Methylpropanoic acid (166.7 g, 1.7 tool), methyl hydrogen adipate (108.8 g, 0.68 mol),
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)