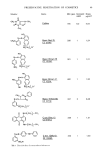
104 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS SE-52. The column temperature was linearly programmed from 100øC to 320øC at a rate of 10øC per min. The injection port and detector temperatures were 300øC. Helium was used as the carrier gas at a rate of 50 ml per min. GC-MS analyses were done with the following conditions: separator temperature, 250øC ion source temperature, 250øC accelating voltage, 3 KV ionization voltage, 70 eV ionization current, 300 microamperes. RESULTS ALLERGENICITY AND PRIMARY IRRITATION OF VARIOUS LANOLIN DERIVATIVES The primary irritation potential of lanolin derivatives to patients with contact dermatitis was 0 to 4.7% (Figure 1). Liquid lanolin solvent • . acetylationAcetylated (8/0/26) fractionation Lanolln I (38/3.1/37) Solid lanolin _• • (8/0/26) • • hydrogenation hydrolysis Hydrogenated lanolin (100/3.1/37) EO addition EO-added hydrogenated lanolin (0/0/26) I Lanolin Lanolin alcohol fatty acid (85/4.7/33) lanolin (8/0/26) (13/1.6/23) •o acetaddition ß n EO Acetylated lanolin EO-added alcohol acetylated (23/0/26) lanolin alcohol (0/0/26) Figure 1. Allergenicity and primary irritation of lanolin derivatives (the values in the figure represent allergic reaction %/primary irritation reaction %/subjects). Allergencity of the derivatives was studied mainly in patients who developed allergic reactions to hydrogenated lanolin, with emphasis placed on a comparison of the reactions due to hydrogenated lanolin. This study has revealed that, although the reaction-positive percentage varies greatly according to the lanolin derivative employed, the addition of ethylene oxide to the derivatives makes their allergenicity disappear. The comparison of the positive percentage of the various derivatives has shown the same results as in the preceding studies, i.e., the allergenicity of the alcohol fractions is high.
ALLERGENS OF LANOLIN 105 Of these derivatives, hydrogenated lanolin indicated remarkably high allergenicity. These findings led us to investigate isolation and identification of the allergens of hydrogenated lanolin. FRACTIONATION OF COMMERCIAL HYDROGENATED LANOLIN In order to determine the chromatographic conditions for isolating the allergens from hydrogenated lanolin, thin layer chromatography (TLC) on silica gel, alumina, and Florisil was carried out. The best separation of hydrogenated lanolin was obtained by TLC on Florisil as shown in Figure 2, where 8-10 spots were observed. Based upon the Solvent f r0nt 0 0 0 o 0o o o : 1 2 3 Figure 2. Thin layer chromatograms of commercial hydrogenated lanolin and its products fractionated by Florisil column chromatography. 1. Commercial hydrogenated lanolin, 2. Benzene fraction of hydrogenated lanolin, 3. Methanol fraction of hydrogenated lanolin. Adsorbent: Florisil, Developer: chloroform. result of TLC, commercial hydrogenated lanolin was separated into two fractions, eluted by benzene and methanol respectively, using column chromatography on Florisil. Thin layer chromatograms of each fraction are shown in Figure 2. Table I shows the results of patch test with these fractions revealing that the allergens of hydrogenated lanolin are present in the methanol fraction and are relatively polar compounds. The allergenic fraction (methanol fraction) was subdivided into four fractions by
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)