410 JOURNAL OF COSMETIC SCIENCE IMPROVMENTS IN SPF CAN BE ACHIEVED THROUGH OPTIMIZATION OF SURFACE FILM INTERACTIONS* Duncan Aust, Ph.D., Vitthal Kulkarni, Ph.D., Joretta Wong, James Wilmott and James Hayward, Ph.D. The Collaborative Group, Ltd., 3 Technology Drive, East Setauket, NY 11733 vitthal. kulkarni @ collabo. com INTRODUCTION Effectiveness of a sunscreen is judged by the sun protection factor (SPF) assigned to it (Cole, 2001). It is well known that depending on the emulsion system and the reproducibility of that system, the same concentrauon of sunscreen agents may produce a wide array of final SPFs. We have consistently produced organic sunscreens using our proprietary high-pressure high-shear technology that have well characterizable nano-particles. Our studies suggest that two fold increase in SPF can be achieved using the nano-dispersions of sunscreens compared to conventional emulsion systems indicating that particle size of the dispersion plays significant role in providing sun protection. MATERIALS AND METHODS A surfactant-free nano-dispersion of sunscreen containing 12% butyl methoxydibenzoylmethane (parsol 1789 La Roche) and 30% of ethykhexyl methoxyciunamate (OMC ISP Van Dyk) was prepared using our proprietary high-pressure high-shear technology and used it at 25% level to produce a finished product. A conven6onal sunscreen containing the same amounts of the organic sunscreen agents was prepared to compare the properties of finished products. In-vivo SPF was determined on a human panel in accordance with the federal regulations. Particle size was measured using Zetasizer 3000 (Malvern Instruments, Inc.). To test the effect of bright sunlight on the sunscreen agents, the two samples (lotion prepared using the nano-dispersion and a conventional lotion) were drawn on glass slides and exposed to natural bright sunlight for a desired period. Active ingredients in the sunscreens were then quantitatively analyzed via HPLC (Jiang et al. 1996). Quality of the film formed by the two sunscreen products, upon rubbing on skin, was studied using a Environmental Scanning Electron Microscope (ESEM) (FeiCo- Phillips, FEG XL-30). The known amounts of sunscreen products were applied on in-vitro skin (IMS, Inc.) and spread on equal area using a finger covered with a finger cot and examined in ESEM. RESULTS AND DISCUSSION Figure 1A shows the in-vivo SPF values of the two sunscreen products. Lotion made with nano- dispersion showed SPF of 15 while the lotion prepared in a conventional manner showed SPF of 8.1 suggesting that particle size, population homogeneity, and spreading behavior may have a significant influence on the ability of the formulated sunscreen actives to protect against the UV radiation. o Figure 1: A: In-vivo SPF for lotions prepared using our proprietary surfactant-fiee nano-dispersion of sunscreens and the 1o6on prepared in a conventional manner •at contained same level of sunscreen actives. B: Average particle size of the two lotions. Particle size of the lo6on made using a nano-dispersion (500-1000 nm, poly index =0.12) is much smaller than that made in a conventional manner (1000 - 3000 nm poly index =1). Size distribution showed that lotion with nano-dispersion had a homogeneous population while the conventional lotion showed poly of 1 suggesting that two or more populations with large difference in size. The particle size of nano-dispersion (the raw material) is 300-600 nm suggesting that no significant change in the particle size occurred for the lotion made from a nano-dispersion. Figure 2 shows the surface of the in-vitro skin model
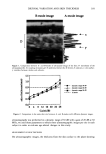
2001 ANNUAL SCIENTIFIC SEMINAR 411 upon application of two lotions. The nano-dispersion lotion showed uniformly spread intact particles (Fig. 2A) where as the regular lotion showed uneven spreading with localized blobs (Fig. 2B). Figure 2 suggests that even after most of the water from lotion evaporates upon application to skin, particles in nano- dispersion lotion remain intact and form a uniform film on the skin surface where as uneven spreading with undefined particles results from application of conventional lotion. This indicates that particles in conventional lotion are susceptible to change in environment. '•. • •' • •'.• • • • - A B Figure 2: Environmental Scanning Electron Micrographs of the in-vitro skin surface applied with sunscreen lotions. A: Lotion with nano-dispersion, B: conventional sunscreen lotion. It is reported that sunscreens formulated with conventional emulsifying agents loose considerable amount of actives (36% loss of parsol 1789) after exposure to UV radiation (Deflandre and Lang, 1988). Figure 3 shows that in nano-dispersion lotion, 75-80% of the sunscreen actives remained intact after 2hrs of exposure to bright natural sunlight while only 60-65% of the actives remained intact in the same period from a conventional lotion. This suggests that a direct relationship exist between UV protecting ability of the sunscreen agents and the size, homogeneity, and stability of the particles after application on skin. Our studies suggest that if a sunscreen formulation spreads on skin to form a uniform film of intact, homogeneous nano-particles it is likely to protect the skin from UV radiation for a considerable time period •roviding a high SPF. • •ø•---•c •a• Figugre 3: Recovery of the z m • exposure to natural bright •0 sunlight. Curvel: From nano- 0 •0 m • ,•0 • dispersion lotion, Curve 2: o 3o •0 •o • •s0 •,,•.•n From conventional lotion CONCLUSIONS ß Nano-dispersion produced by proprietary technology showed uniform particle size in the range of 300- 600 nm ß Our studies showed that lotion made from nano-dispersion spread uniformly on skin compared to the lotion of the same ingredients made in a conventional manner ß Our studies indicated that the integrity of the sunscreen actives is strongly dependent on the formulation. Actives from nano-dispersion lotion were significantly stable compared to the same actives from lotion made in a conventional manner ß Our studies suggest that formation of a uniform film with intact nano-particles is the key for achieving nearly two-fold SPF improvement over conventional lotion REFERENCES Cole, C., (2001) "Sunscreen protection in the ultraviolet A region: how to measure the effectiveness" PhotodermatoL Photoimmunol. Pho toreed. 17:2-10 Deftandre, A, and Lang, G. (1988) "Photostability assessment of sunscreens. Bcnzylidcnc camphor and dibcnzoylmcthanc derivatives" Intl. J. Cosmet. Sci. 10, 53-62 Jiang, R., Hay&n, C.G., Prankerd, R.J., Roberts, M.S., and Benson, H.A. (1996) "High-performance liquid chromatography assay for conunon sunscreening agents in cosmetic products, bovine serum albumin solution and human plasma" J. Chromatogr. B Biotaed. AppL 682, 137-145. * This paper is being presented by Jennifer Corwin on behalf of The Collaborative Group.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)