412 JOURNAL OF COSMETIC SCIENCE SUNSCREEN PHOTOSTABILITY AND UVA PROTECTION Joseph W. Stanfield Suncare Research Laboratories, LLC, 740 East Brookhaven Circle, Memphis, TN According to the FDA Sunscreen Monograph, the sun protection factor (SPF) of a sunscreen is measured in vivo on the skin of human volunteers using a solar simulator. The solar simulator UV spectrum resembles that of sunlight at high sun angles, which occur in mid-summer. At lower sun angles the actual SPF of a sunscreen in sunlight is lower than its labeled value, unless the product provides "broad spectrum" protection, that is, a substantial level of UVA protection. In addition, if the sunscreen is not photostable, its SPF and UVA protection may diminish rapidly in sunlight. This presentation describes an m vitro method for assessing sunscreen UVA protection and photostability. Since many sunscreen formulas are not photostable, any method for assessing UVA protection must take into account the photostability of the product, to be valid. In vitro measurements of sunscreen protection simulate the in vivo SPF test by using a measured amount of sunscreen applied to an artificially prepared substrate, instead of living skin. The SPF of the substrate and the SPF of the sunscreen are determined by measuring the UV transmission of the substrate alone and the substrate containing the sunscreen, as shown below: o 'Is Ess Sunscreen Substrate I0 is the effective UV irradiance applied to the surface of the substrate alone and Is is the effective UV irradiance transmitted by the substrate alone. Io and Is are obtained by measuring spectral irradiance values from 290 to 400 nm, multiplying by the CIE erythemal effectiveness spectrum and integrating over wavelength to calculate irradiance. The SPF of the substrate, SPFs = IdIs, and is assumed to be constant. E0 is the measured effective UV dose from 290 to 400 nm applied to the surface of the sunscreen on the substrate Ess is the measured effective UV dose from 290 to 400 nm
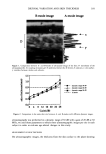
2001 ANNUAL SCIENTIFIC SEMINAR 413 transmitted through the combined sunscreen and substrate and E is the effective UV dose transmitted through the sunscreen only, computed as E = SPFs *Ess. Eo, Ess, and E are irradiance values integrated over time, and expressed in multiples of the MEI} (0.02 effective joules/cm2), which is the UV dose required to produce minimally perceptible erythema (sunburn). Measured values of Eo are plotted against computed values of E, and the SPF of the sunscreen at any given time is Eo / E. The SPF measured in the in vivo SPF test is the value of Eo when E reaches one IVIED. The value of SPF measured in vitro must be approximately equal to the SPF measured using the in vivo test for the assessment of photostability and UVA protection to be valid. A power curve fit equation, E =ctEo I•, may then be computed using a least squares curve fitting method, as shown below for a typical broad spectrum sunscreen product with an SPF of 26: 3 E = 0'0098Eø"4's4 • •= R 2 = 0.99 • g • SPF 0 10 20 30 40 50 60 Eo (Effective UV Dose Applied, MED) Since a value of 1 for [3 represents a linear relationship between E and Eo, and a constant SPF, a sunscreen may be considered photostable if the value of I• is near 1. In the example shown above, I• = 1.4184, and the sunscreen is not considered photostable. We have set a proposed limit of 1.1 for It. Thus, if [• 1.1, a sunscreen may be labeled as photostable. The UVA protection factor, APF, represents the UVA component of SPF. APF may be calculated in the same manner as SPF, for the wavelength range from 320 to 400 nm There is general agreement that the contribution of UVA to the acute effects of sunlight is about 20%. Therefore, if the ratio of APF to SPF is at least 20 percent, we propose that the sunscreen may be labeled "Broad Spectrum."
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)