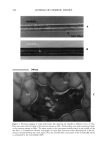
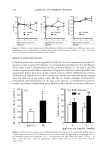
468 JOURNAL OF COSMETIC SCIENCE 1,2 -0,2 Temperature (øC) Figure 1. DSC curves of the developed SLN formulations (A, B, and C) recorded seven days after sample production. the viscous and elastic behavior of the investigated systems and the network structure formed by particle-particle interactions, an oscillation frequency sweep test has to be performed (13). When performing oscillation measurements, first the linear viscoelastic region has to be determined by a stress sweep at a constant frequency. The linear viscoelastic region is the range of stress over which G* is independent of the applied stress. Over this linear region the structure of the dispersion remains intact. The stress sweep test is a dynamic test in which the complex modulus G* is measured as a function of stress at a constant frequency. Being in the viscoelastic region for all tested formulations, 5 Pa was chosen as the stress amplitude in the following studies. The shape of the material function curves reveals structural characteristics of the system. Therefore, in the present work this test was performed over a frequency range from 0 to 10 Hz. Figure 2 displays the storage modulus (G'), loss modulus (G"), and complex viscosity (Xl*) of the developed SLN formulations (A, B, and C), after application of a frequency range between 0 and 10 Hz. With regard to formulation A (SLN based on Softi- san©138), it can be seen that G' is lower that G", which means that the system is more viscous than elastic. For both B (SLN based on Compritol©888) and C (SLN based on stearyl alcohol) formulations, G' is higher by about one order of magnitude than G", and so the system is more elastic than viscous in the investigated frequency range and both parameters show weak dependence on the applied frequency. Note that formulation A is a supercooled melt it has a lower Xl* and shows less suitability for topical adminis- tration because it might readily flow out of the container. Conversely, formulations B and C are highly crystalline and show a prominent elastic component. Another reason for the obtained differences is the percentage of lipid phase in each formulation. In more concentrated dispersions (B and C), relatively large amounts of lipid may significantly increase the effective volume fraction of the dispersion, and hence the viscosity, due to the quantity of immobilized liquid between the lipid phase and the relatively high surface area of the nanoparticles. According to these results, formulations B and C might
VISCOELASTICITY OF SLN AND NLC FORMULATIONS 469 [Pal U'-'-'-q •' [Pa] q* [mPas] I 250 200 ß •. 150 • 100 5O A B C Formulation 4500 4000 3500 3000 2500 2000 1500 1000 50O 0 Figure 2. Storage modulus (G'), loss modulus (G"), and complex viscosity 0q*) of the developed SLN formulations, after application of a frequency range between 0 and 10 Hz. be suitable for topical administration because they exhibit a more uniform resistance against flow. Both G' and G" moduli, as well as qq*, are also affected by the type and concentration of the surfactant in the formulation (14). Formulation B is sterically stabilized with poloxamer and, to provide a sufficient steric hindrance, a relatively large amount of this polymer is used, which might increase the effective volume fraction, resulting in higher viscosity. Therefore, the influence of the oil used for NLC preparation in its viscoelastic parameters has been evaluated in comparison to the respective SLN system. Regarding the obtained G' and G" values for NLC formulations, a completely different rheological behavior can be observed as a function of the liquid lipids of the formulations. The results are shown in Figures 3 and 4. Using Compritol©888 as solid lipid, Miglyol©812 (B L) revealed a G" value four times higher than G', and therefore this oil is responsible for a more viscous system. In contrast, when using stearyl alcohol (C1), both moduli lie in the same range of mag- nitude. Tocopherol showed a greater influence on elasticity when formulated with stearyl alcohol as a solid lipid in NLC (C2). Sunflower oil also displayed significant differences with regard to the solid lipid. In fact, when using Compritol©888 (B3) , the G' was twice as high as G", showing that the system is more elastic than viscous, but the same was not detected when using stearyl alcohol (C3). Concerning LCT, this oil did not reveal any significant difference with regard to the solid lipid used for NLC prepa- ration (8 4 and C4). Regarding the obtained qq* values, formulations B 2 and B 4 indicated a significant decrease in viscosity in comparison to the SLN system (Figure 3). Stearyl alcohol-based formulations (Figure 4) showed higher values, although the influence of the liquid oils was more pronounced in Compritol©888-based formulations (Figure 3). The lower zeta potential values of the stearyl alcohol-based nanoparticles might be responsible for the higher qq* values recorded for these systems. In fact, the decrease in electrostatic repul- sion between the suspended nanoparticles might be responsible for the develop-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)