M. HETEROPHYLLA AND INHIBITION OF MMP-1 283 ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA) The expression level of MMP-1 was determined by the ELISA method as described previously (11). Briefly, after incubation for 24 h, the supernatants of UV-irradiated cultures were transferred to a 96-well plate, and coating buffer (Na2CO 3 1.59%, NaHCO 3 2.93%, NaN 3 0.20%, MgC12 1.02%, pH 9.6) was added at the same volume and incubated for 24 h. The coated wells were washed with PBS containing 0.05% Tween 20 (PBS-T), followed by blocking with 3% BSA in PBS-T for 1 h at 37°C. After washing the wells, 0.3 µg/ml of a monoclonal anti-MMP-1 antibody in PBS-T was added to each well and incubated for 60 min. After washing the wells, 0.2 µg/ml of goat anti-mouse IgG conjugated with alkaline phosphatase in PBS-T was added and incu bated for 60 min. After washing the wells, 100 µl of 1 mg/ml p-nitrophenyl phosphate (pNPP) in a diethanolamine buffer was added for 30 min. The optical density was measured at 405 nm using an automated microplate reader. Finally, the cytotoxicity of the supplemented samples was measured by the MTT assay (12). STATISTICAL ANALYSIS All experiments were performed in triplicate. Data were presented as mean±standard deviation (SD). Experimental results were statistically analyzed by using the Student's t-test (SigmaPlot 2000). All p values less than 0.05 were considered statistically sig nificant. RESULTS AND DISCUSSION Activity-guided column chromatographies of an EtOAc soluble fraction from extracts of M. heterophylla led to the isolation of two compounds. Compound 1 was obtained as an amorphous pale brown powder and reacted positively to the ferric chloride test. The molecular formula was determined to be C 34 H2 8 O22 by FAB-MS, which showed a quasi-molecular ion peak at m/z 789 [M+ + 1-}. The UV spectrum exhibited maxima at 217 and 277 nm. In the 1 H-NMR spectrum, four singlet signals of aryl protons of galloyl groups were detected at B 7 .26-7 .32. A typical anomer H signal was found at 6.10 ppm (lH, d,J:::: 9.3 Hz) and the other sp3 protons of sugar moiety were detected at 4.41-5.45 ppm. In the 13 C-NMR spectrum, the signals of four carbonyl carbon were shown at 167.32 (lC), 167.13 (2C), and 166.94 (lC) ppm, respectively. Twenty-four aromatic carbons of four galloyl groups were detected at 146.27-146.3 7 (SC, C-3 ', 5 '), 139.00-139.29 (4C, C-4'), 121.77-122.18 (4C, C-1 '), and 110.26-110.42 (SC, C-2', 6') ppm. The anomer C signal and five sp3 carbons were detected at 102.93 and 63.08-74.02, respectively. From these data, compound 1 was postulated to be 1,2,4,6- tetra-O-galloyl-�- 0 -glucopyranose (Figure 1). The structure was verified by the reported spectral results (13-16). Compound 2 was obtained as an amorphous white powder and reacted positively to the ferric chloride test. The molecular formula was determined to be C 7 H 6 O 5 by FAB-MS, which showed a quasi-molecular ion peak at m/z 171 [M+ + 1}. The UV spectrum exhibited maxima at 220 and 272 nm. In the 1 H-NMR spectrum, only one peak was detected, at 7 .15 (2S, s, H-2, 6). The signal of carbonyl carbon appeared at 167. 86 ppm, and six aromatic carbons were detected as four peaks at
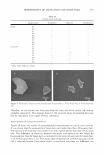
284 JOURNAL OF COSMETIC SCIENCE HO HO�oH o=c HO b OH HO �c-o�Q }-i-oH Y-8 �o � HO OH I OH Ao HOYoH OH COOH HO OH OH Compound 1 Compound 2 Figure 1. Chemical structures of compounds 1 and 2 isolated from M. heterophylla. 110.31-146.07 ppm in the 13 C-NMR spectrum. The final structure was identified as 3,4,5-trihydroxybenzoic acid (Figure 1) by referring to the reported data (17). We studied the antioxidant effects of two compounds from M. heterophylla using DPPH and superoxide radicals. These compounds were found to have a strong antioxidant activity. Compounds 1 and 2 exhibited a potent scavenging activity against the DPPH radicals, with SC 50 (the concentration of the sample required for 50% of the free radicals to be scavenged) values of 3.9 µM and 13.3 µM, respectively. Furthermore, compound 1 appeared to be the most efficient in comparison with the three reference compounds, vitamin C (SC 50 60.0 µM), vitamin E (SC 50 30.0 µM), and epigallocatechin-3-gallate (EGCG, SC 50 6.4 µM), which is well known as a scavenger of DPPH radicals (Table I) (18,19). Also, compounds 1 and 2 exhibited a potent scavenging activity against the superoxide radicals in the xanthine/xanthine oxidase system, with SC 50 values of 4.3 µM and 4.0 µM, respectively, compared with butylated hydroxyanisole (BHA, SC 50 Table I Antioxidant Effects of Compounds 1 and 2 Isolated from M. heterophylla SC50a values (µM) Compounds DPPH6 Superoxide anion C 3.9 4.3 2 13.3 4 Vitamin C* 60 NDd Vitamin E* 30 NDd EGCG* 6.4 4.9 BHA* NDd 180 a Concentration giving a 50% decrease of DPPH and superoxide radicals. The values are the means of triplicate experiments with SD. 6 1,1-diphenyl-2-picrylhydrazyl radical. c Superoxide anion radicals were produced from xanthine/xanthine oxidase oxidation system. cl Not determined. * Used as a positive control.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)