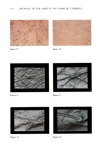
448 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 3.5 3.0 2-5 2.0 1.5 1.0 0'5 .,•106 KCI NaCI CaCI. ALL IONS NaHCO 3 0 i I I 50 60 70 80 90 TEMPERATURE (øC) Figure 7. Isometric melting curves of rat tail tendon in O.15M NaC1, after 6 months in vitro aging in solutions of O.15M NaC1 in various salts. Each salt was present at its "physiological" concentration (see text). The salt written against each curve indicates that the incubating solution was O.15M NaCl plus this salt. than does type I, and these two types are associated with hyaluronic acid and dermatan sulphate, respectively. Hyaluronic acid binds large amounts of water, and tissues which contain predominantly type III collagen are gel-like. All these arrangements are age-related type III collagen, which is prominent in fetal skin, decreases in amount with respect to type I after birth and continues to decrease with age. This change may be related to the "drying" of skin with age.
AGING OF COLLAGEN 449 There is accumulating evidence that the connective tissue cell responds to physical and mechanical stimuli in its environment. A good example is that of the rabbit flexor digitorum profundis tendon, which curves around the back of the ankle (28). On the pressure side the glycosaminoglycan content is --•3% of which 60% is chondroitin sulphate, while in the tensional parts of the tendon glycosaminoglycan content is --•0.2% of which 70% is dermatan sulphate. If the tendon is translocated so that it is subjected only to tensional forces, chondroitin sulphate is gradually replaced by dermatan sulphate. The whole process can be reversed. Thus, some forms of pathological aging may be the result of a normal response of the cell to an unnatural stimulus, e.g., gross mechanical stress. KERATIN The outer layer of the skin of vertebrates, the epidermis, produces a range of fibrous proteins collectively termed keratins (29). Specialized epidermal cells also give rise to the follicle, the complex structure in mature skin which produces the keratin fiber, hair and wool. Keratin proteins, in contrast to collagen, are laid down intracellularly and they characteristically contain cysteinyl residues which are oxidized in the final stages of biosynthesis to form a network of intra- and inter-molecular disulphide cross-links. The disulphide links are the counterpart of the aldimine links of collagen, but whereas the aldimine links alter with in vivo aging the disulphide link undergoes no further maturative changes. It is a strong bond responsible for the characteristic insolubility and mechanical toughness of keratin fibers. However, keratin of the stratum comeurn, in particular, undergoes a constant degradation and replacement cycle due mainly to mechanical abrasion and ultra-violet irradiation this is its function as the front line protector of the body. Hair and wool fibers exhibit maturative aging for some time after emerging from the follicle. It has been shown recently (30) that stress-relaxation and water swelling properties of keratin fibers are different from root to tip: beginning at the root-end the stress-relaxation decreases, while the water-swollen diameter increases as one samples toward the tip. This pattern changes with time, the root-end becoming more tip-like until in old fibers (stored in air for at least 3 years) the properties are uniform along the fiber. The process correlates with a gradual decrease in the thiol content of samples taken progressively toward the tip-end. Atmospheric oxidation is almost certainly responsible for the process, because it can be prevented in the laboratory by storing the fiber under nitrogen. CONCLUSION In vitro studies help in the understanding of some of the processes involved in the in vivo maturation and aging of collagenous tissue. First, they strongly suggest that oxygen is intimately involved in the increases in mechanical and thermal stabilization of tissues with age, possibly by reacting with the initially formed reducible aldimine cross-links to form stable multi-functional links. Secondly, in vitro studies support the view that ascorbic acid has a stabilizing effect upon newly-synthesized and "young" tissue. Thirdly, the inorganic ions normally resident in tissue fluid directly affect the stability of tissue over time, and may be expected to behave likewise in vivo.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)