250 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS invasion by foreign substances, is now acknowledged as a highly complex organ through which many substances can diffuse or be transported. The significance of this fact has become more evident largely through the research efforts of an increasing number of investigators involved in studies on transdermal permeation of various drugs. Many consider this to be the optimal mechanism for the design of drug delivery systems. In such systems the skin may be the rate-limiting factor in others, skin diffu- sion is sufficiently high that synthetic polymers are needed to provide the rate-control- ling function. Several examples of systemically active transdermal drug delivery systems are presently available and many others are under investigation. The research cited above has heightened the awareness of many investigators and regula- tory agencies to the potential advantages as well as risks of exposure to a variety of environmental substances, including those used in cosmetic formulations. The review of over-the-counter (OTC) drug ingredients and claims by the Food and Drug Adminis- tration (FDA), which began in 1972 (1-3), has had a profound impact on the cosmetics industry. The FDA/OTC review has resulted in the removal from the market of ingre- dients used in cosmetic products (e.g., hexachlorophene) or has modified the testing procedures and label claims (e.g., ultraviolet light-absorbing agents used as sunscreens) (4). In response to these actions the Cosmetic Ingredient Review Program (CIR) (5) was established by the cosmetics industry to ascertain the safety, or lack of safety, of ingre- dients used in cosmetic products. There are many problems in evaluating the effectiveness and potential toxicologic prop- erties of topically applied substances. Some of these are: 1) lack of a non-human animal model with skin which closely resembles that of the human, 2) lack of uniformity in skin characteristics between different human subjects and even skin from different sites on a single human subject (6,7), 3) lack of an in vitro model system which approximates closely the in vivo characteristic of human skin, 4) lack of uniformity in sensitivity and allergic reactions to applied substances, and 5) the unacceptability of using human subjects for evaluating potentially toxic or dangerous substances. Recent publications by Elias et al. (6), and Handjani-Vila et al. (7) have demonstrated that structural fea- tures and lipid content of stratum corneum have greater impact on percutaneous trans- port of topically applied substances than does the thickness of this component of the skin. Qualitative variations of transcellular, intracellular, and appendageal transport also represent unresolved questions concerning percutaneous absorption of applied sub- stances. During the mid 1970s, techniques for transplantation of human skin onto the congeni- tally athymic (nude) mouse were reported (8,9) and the transplanted skin shown to retain the histologic features of the donor (10, 11). It was demonstrated that the trans- planted skin would exhibit characteristic growth of epidermal appendages (9, 12). Krueger eta/. (13,14) further demonstrated that pig skin transplanted onto the nude mouse retained its pretransplant properties relative to proliferation and barrier func- tions. In 1981 Krueger and Shelby (15) further substantiated the maintenance of donor qualities of the grafted skin by exposure of the graft to agents which modify epidermal proliferation, e.g., 12-O-tetradecanoyl-phorbol- 13-acetate (TPA). Reifenrath eta/. (16) compared several animal models, including the human skin grafted athymic nude mouse and pig skin grafted athymic nude mouse, for their trans- dermal absorption of nine topically applied radiolabeled compounds. These investi-
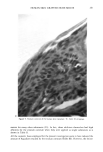
HUMAN SKIN GRAFTED NUDE MOUSE 251 gators concluded that a significant correlation existed between the human skin/nude mouse and human values, whereas no significant correlation existed between human values and those of pig skin/nude mouse or hairless dog values. Due to several factors, including those enumerated above, the availability of the animal model and the expertise required for a highly interdisciplinary study, it was concluded that a study should be conducted to develop the methodology for utilizing the human skin/nude mouse as a model for evaluating the uptake, transport, and retention of selected cosmetic ingredients by the stratum corneum. METHODS GRAFTING OF MICE The mice used in this study were obtained from a colony maintained by Gerald G. Krueger, M.D., Division of Dermatology, University of Utah. The colony was origi- nated by mating pathogen-free female Balb/c mice heterozygous for nude with Balb/c males homozygous for nude (15). Because the animals are athymic they must be main- tained in a pathogen-free environment. Otherwise, standard maintenance procedures were utilized. Details on animal maintenance, skin harvesting, storage, and grafting procedures have been described in previous publications (8-15). The human skin used for transplantation was obtained from females undergoing abdominoplasty. Skin was obtained shortly after surgery and was dermatomed to about 0.4 mm thickness and kept at 2-5øC in RPMI1640 (Flow Labs. Inc., Virginia) to which was added 10% Fetal Bovine serum (FBS) (Flow Labs, Inc.). Skin maintained under these conditions could be used for transplantation for up to five days. The skin samples were cut into square sections approximately 1.5 cm per side. Grafting was accomplished by anesthetizing nude mice about 11- 16 weeks of age with intraperitoneal injection of 97.5 mg/kg of 4% chloral hydrate solution, clipping away a section of the mouse's skin behind the left front leg, placing the human skin over the denuded area, and bandaging the area. Under these conditions vascularization of the graft requires about 3-5 days and healing is complete in about three to four weeks. EXPERIMENTAL TECHNIQUES Autoradiographic analyses. In order to assess the uptake and permeation of the radiola- beled chemicals, autoradiography was chosen as a preliminary study. Employing this technique, the treated sections of skin were first cleansed with a mild aqueous soap solution and cotton swab. Punch biopsies 4 mm in diameter were then taken, placed in OCT Fluid (Tissue Tech. Tissue Tech II OCT Mounting Fluid), and immediately frozen in liquid nitrogen. Frozen samples were cut into thin crossections (about 6 •m) with a cryostat (American Optical Model 975C histostat cryostat) and mounted onto glass slides. The sections were then autoradiographed by standard techniques. Briefly, this includes coating the slide with a special photographic emulsion (Eastman Kodak, Nuclear Track Emulsion NTB-3) allowing several (2-5) days exposure, then devel- oping and fixing the exposed slide. The resulting slides were photomicrographed in
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)