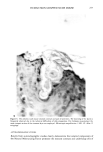
270 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS RESULTS AND DISCUSSION An initial screening of a shampoo a for volatile nitrosamines revealed a relatively large response on the GC/TEA at the retention time characteristic of NMDDA. Based upon external standard calculations, the apparent NMDDA concentration was approximately 12 ppm. This level was unexpectedly high since literature values for volatile nitrosa- mines in shampoos are at the 10-200 ppb range (2), and since the product contained none of the likely NMDDA precursors. GC/MS analysis of the shampoo extract for NMDDA utilizing a non-polar 30m DB-1 capillary column revealed the reconstructed ion chromatogram (RIC) shown in Figure 1. The chromatogram is complex and largely dominated by an overloaded component appearing at scan 1881. This peak was identified as myristic acid (extracted from the shampoo) along with some coeluting minor components which will be discussed later. A GC/MS analysis of NMDDA was also performed and its mass spectrum is shown in Figure 2. The NMDDA spectrum contains two primary fragment ions at masses 211 and 198, with a relative intensity ratio of 3/1, respectively. Additionally, the NMDDA standard coeluted with the myristic acid on the DB-1 column. Utilizing the single-ion monitoring capability of the GC/MS, it is possible to observe Shampoo Extract 100.0 - RIC '' ' ' I ' ' ' oel I ' I I I ' il I ' ' ' ' I ' ' ' I I ' ' ' I ' ' ' '1 500 1000 1500 2000 2500 3000 3500 4000 SCAN 4:35 9:10 13:45 18:20 22:55 27:30 32:05 36:40 TIME Figure 1. Reconstructed ion chromatogram of a shampoo extract on a 30 m DB-1 capillary column by electron impact ionization. a Shampoo ingredients: water, ammonium lauryl sulfate, sodium lauryl sulfate, fragrance, myristic acid, SD alcohol 40, glycol distearate, cocamide DEA, disodium phosphate, EDTA, sodium hydroxide, am- monium chloride, methylchloroisothiazolinone, methylisothiazolinone, D&C Green No. 8, and FD&C Blue No. 1.
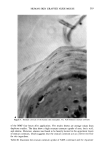
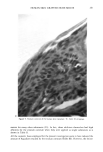
FALSE POSITIVE NITROSAMINE ANALYSES 271 NMDDA 100.0 - 43 50.0 74 211 61 198 - I , , ,, I II, 111 168 I .... • ,', '1 .... t .... [" "•'" '1 .... I .... I' "'1" "1" "•' "'1 ""l' •' I .... • .... I 60 80 1 O0 120 140 160 180 200 220 M/Z 40 Figure 2. Electron impact mass spectrum of a standard 88 ng/•l NMDDA solution 4 •l splitless injec- tion. only selected ions specific for a compound(s) of interest. Figure 3a shows a series of these single ion chromatograms called "mass chromatograms" from scan 1700-2100 for a 0.5 •1 splitless injection of the shampoo extract at masses 198 and 211, indicative of NMDDA, and mass 228, which is the molecular weight of myristic acid. Figure 3b shows a 1 •1 splitless injection of the same shampoo extract which was spiked with 3 •1 of an 88 ppm NMDDA standard. Note the appearance of a new peak in the single ion chromatograms for masses 198 and 211 (at scans 1861/1862) which were not present in the 198 and 211 single ion chromatograms of the neat shampoo extract (Figure 3a). Although the NMDDA standard does coelute with the myristic acid, the single ion monitoring capability makes it possible to observe the NMDDA in the spiked sample which does not appear in the neat shampoo extract. A new peak also appeared at scan 2026 (mass 198) due to an impurity in the NMDDA standard. As a further confirmation that the shampoo extract did not contain NMDDA and that the TEA signal was a "false positive," a UV-photolysis confirmatory experiment was performed on the shampoo extract. Nitroso compounds (e.g., N-nitrosamines) are not stable to UV radiation while nitro compounds are stable (8). Upon irradiation of the extract followed by reanalysis with the GC/TEA, disappearance of the TEA response indicates the presence of a nitroso compound while no change in the TEA signal indi- cates the presence of a nitro compound (8). UV irradiation of the shampoo extract did not alter the TEA signal. This observation supports the GC/MS results indicating no NMDDA was present in the shampoo extract.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)