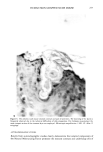
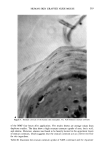
276 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS a. 6.4 211 Shampoo Extract 100.0 228 15.5 278 6.2 297 RIO 1200 1300 1400 1500 1600 1700 1800 1900 2000 SCAN 11:00 11:55 12:50 13:45 14:40 15:35 16:30 17:25 18:20 TIME b. 5.6 211 100.0 228 Shampoo Extract Spiked With NMDDA 14.9 278 6.4 297 RIO [ I [ • I ' [ • I ' I ' [ I [ ] I I [ I /[ [ I I , I ' I 1200 1300 1400 1500 1600 1700 1800 1900 2000 SCAN 11:00 11:55 12:50 13:45 14:40 15:35 16:30 17:25 18:20 TIME Figure 7. (a) Mass chromatograms for a 0.2 p•l on-column injection of a shampoo extract at masses 211 (NMDDA), 228 (myristic acid), 278 (moskene), and 297 (musk xylol). The reconstructed ion chromato- gram is also shown. (b) Mass chromatograms and reconstructed ion chromatograms as described above for a 0.2 p•l on-column injection of a shampoo extract spiked with 0.3 p•l of a standard 88 ng/p•l NMDDA solution. Note the appearance of a new peak at scan 1510 for mass 211 in the spiked sample which is now well separated from the nitromusks and the myristic acid. This separation was carried out on a 30m DX-4 polar capillary column.
FALSE POSITIVE NITROSAMINE ANALYSES 277 persisted after UV-photolysis. In addition, the musk xylol retention time could not be distinguished from that of the NMDDA standard. In order to improve the quality of the GC/MS (and potentially GC/TEA) analysis for nitrosamines in the presence of nitromusks and myristic acid, a better separation scheme was developed. This method utilized a 30m DX-4 polar capillary column and on-column injection to greatly improve the separation of these materials. Figure 7a shows a series of single ion chromatograms at masses 211 (NMDDA), 228 (myristic acid), 278 (moskene), and 297 (musk xylol) for a 0.2 ptl on-column injection of the neat shampoo extract. Figure 7b shows the same single ion chromatograms for the same amount of extract spiked with 0.3 ptl of the 88 ppm NMDDA standard. Note the absence of a peak at mass 211 in the neat shampoo extract and its appearance (scan 1510) in the spiked sample. Also note that the NMDDA is well separated from the nitromusks and from the myristic acid. This separation technique could be adapted for the GC/TEA systems which would eliminate this false positive problem when using the GC/TEA. We would like to stress, however, that confirmatory experiments are still necessary when using the GC/TEA. CONCLUSION The TEA is an excellent detector for determination of nitrosamines providing its limita- tions are understood. Because of the high specificity of the TEA, it is often used blindly and the required confirmatory experiments such as GC/MS, UV-photolysis, or wet chemical procedures are neglected. Special attention should be given to the determination of nitrosamines in any fragranced product due ro the widespread use of nitromusks in perfumes which may produce false positive results when using the TEA. ACKNOWLEDGEMENTS We would like to acknowledge Thermedics, Inc. (Woburn, MA) and Hazleton Labora- tories America (Madison, WI) for the GC/TEA analyses. REFERENCES (1) T. Y. Fan, E. U. Goff, L. Song, D. H. Fine, G. P. Arsenault, and K. Bieman, N-nitrosodiethanol- amine in cosmetics, lotions, and shampoos, Food Cosmet. Toxicol., 15, 423-430 (1977). (2) S. S. Hecht, J. B. Morrison, and J. A. Wenninger, N-nitroso-N-methyldodecylamine and N-ni- troso-N-merhylretradecylamine in hair-care products, Fd. Chem. Toxicol., 20, 165-169 (1982). (3) M.D. Erickson, D. B. Lakings, A.D. Drinkaine, and J. L. Spigarelli, Determination of N-nitroso- dierhanolamine (NDELA) in cosmetic ingredients, J. Soc. Cosmet. Chem., 36, 223-230 (1985). (4) D. H. Fine and D. P. Rounbehler, Trace analysis of volatile N-nitroso compounds by combined gas chromatography and thermal energy analysis, J. Chromatog., 109, 271-279 (1975). (5) D. H. Fine, F. Rufeh, and B. Gunther, A group specific procedure for the analysis of both volatile and non-volatile N-nitroso compounds in picogram amounts, Anal. Lett., 6, 731-733 (1973). (6) T. Y. Fan, I. Krull, M. Wolf, R. Ross, and D. H. Fine, "Comprehensive Analytical Procedures for the Determination of Volatile and Non-Volatile, Polar and Non-Polar N-Nitroso Compounds," Pro- ceedings of the 5th IARC Meeting on the Formation and Analysis of N-Nitroso Compounds, Durham, NH, (1977). (7) T. Y. Fan, R. Vita, and D. H. Fine, C-nitro compounds: A new class of nitrosating agents, Toxicol. Lett., 2, 5-10 (1978). (8) I. S. Krull, E. U. Goff, G. G. Hoffman, and D. H. Fine, Confirmatory methods for the thermal energy determination of N-nitroso compounds at trace levels, Anal. Chem., 51, 1706-1709 (1979).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)