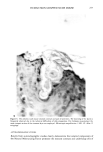
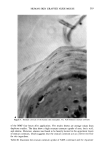
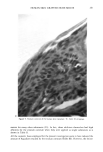
264 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS nude mouse is a useful model for evaluating the uptake, transdermal permeation, and possibly the desorption characteristics of radiolabeled substances. This would appear to offer advantages of great importance to the cosmetics industry in terms of assessing the potential toxicologic properties created by transdermal uptake of cosmetic ingredients as well as the evaluation of claims for preparations designed to have a beneficial effect on various segments of the epidermis. There are certain aspects of the procedure which limit its usefulness. Since only a frac- tion of a litter from the heterozygous/homozygous parents are nude, the animals are quite expensive. Access to live human skin, grafting procedures, autoradiographic pro- cedures, microtoming procedures, handling of radiolabeled chemicals, and other aspects of the total study involve personnel with diversified skills. Thus, a large interdisci- plinary effort is essential. The necessity of maintaining the animals in a pathogen-free environment places further limitation on the procedures. Since these procedures utilize radiolabeled chemicals, availability of these substances is critical. Custom-labeling is possible but expensive if stock items are not available. It should also be mentioned that the grafting procedures do not assure that 100% of the animals will be useful. About 90-95% of the grafted animals survive however, some of the grafts tend to shrink and appear to scar. These animals should not be used. Skin pigmentation also occurs but does not appear to affect test results. Despite the disadvantages, the procedure has obvious advantages and should be consid- ered a viable method, or even method of choice, since it offers the advantage of testing substances on functional human skin without exposing humans to toxicologic or ra- diologic hazards. ACKNOWLEDGMENTS The authors express gratitude for the enthusiastic participation and cooperation of the following persons: Gerald Krueger, M.D., (Dermatology) for sharing his experience in grafting techniques Dr. Jerry Nelson and Mr. Taylor Davis (MIDECO Laboratories) for maintaining the animals and performing various tests Susan Wall (Pathology) for mi- crotoming frozen sections Dr. Donald E. Gregonis for assistance with chemical syn- theses Dr. William I. Higuchi, Dr. Sung-Wan Kim, and Dr. Dennis L. Coleman, for their constructive suggestions and advice and Kolmar Laboratories, Inc., for their fi- nancial support of the project and participation in preparing and providing Natural Moisturizing Factor. REFERENCES 1. 37 Federal Register, 85 (January 5, 1972). 2. 37 Federal Register, 9469 (May 11, 1972). 3. 37 Federal Regzster, 1474 (May 11, 1972). 4. R. R. Giovacchini, The impact of the OTC drug review on cosmetic safety substantiation, Pharm. Tech. Conf. 83, New York, September, 1983, p 59. 5. R. Giovacchini, The cosmetic ingredient review program, Food Drug Cosmetic Law Journal, 32, 40-43 (1977). 6. P.M. Elias, E. R. Cooper, A. Korc, and B. E. Brown, Percutaneous transport in relation to stratum comeurn structure and lipid composition, J. Invest. Derre., 76, 297-301 (1981).
HUMAN SKIN GRAFTED NUDE MOUSE 265 7. R. M. Handjani-Vila, A. Rabier, B. Rondot, and G. Vanlerberghie, Dispersion of lameIlar phases of nonionic lipids in cosmetic products, Int. J. Costa. Sci., 1, 303-319 (1979). 8. N. D. Reed and D. D. Manning, Long-term maintenance of psoriatic human skin on congenitally athymic (nude) mice, Proc. Soc. Exp. Bio/. Med., 143, 350-356 (1973). 9. J. Rygaard, Skin grafts in nude mice, Acta Path. Microbio/. Scand., 82, 105-112 (1974). 10. G. G. Krueger, D. D. Manning, J. Malouf, and B. E. Ogden, Long-term maintenance of psoriatic human skin on congenitally athymic (nude) mice, J. Invest. Dermato/, 64, 307-312 (1975). 11. R. A. Briggaman and C. E. Wheeler, Jr., Lameliar itchthyosis: Long-term graft studies on congen- tially athymic nude mice, J. Invest. Dermato/., 67, 567-576 (1976). 12. D. D. Manning, N. D. Reed, and C. F. Shaffer, Maintenance of skin xenografts of widely divergent phylogenetic origin on congenitally athymic (nude) mice.,J. Exp. Med., 138, 488-494 (1973). 13. G. G. Krueger, D. A. Chanbers, andJ. Shelby, Epidermal proliferation of nude mouse skin, pigskin, and pig skin grafts,J. Exp. Med., 152, 1329-1359 (1980). 14. G. G. Krueger, D. A. Chambers, and N. J. Shelby, "Comparative Effective of Proliferative Agents on Nude Mouse Skin, Pig Skin and Pig Skin Xenogeneic on Nudes," in Proc. Third Int. Workshop on Nude Mice, (Gustav Fisher, New York, Stuttgart, 1982), Vol. 2, pp 435-445. 15. G. G. Krueger, and J. Shelby, Biology of human skin transplanted to the nude mouse: I. Response to agents which modify epidermal proliferation, J. Invest. Dermato/., 6, 501-511, 1981. 16. W. G. Reifenrath, E. M. Chellquist, E. A. Shipwash, W. W. Jederberg, and G. G. Krueger, Per- cutaneous penetration in the hairless dog, weanling pig and grafted athymic nude mouse evaluation of models for predicting skin penetration in man. Brit. J. Dermato/., Supp. 27, 123-135, III, 1984. 17. O.K. Jacobi, U.S. Patent 3,231,472 (Jan. 25, 1966). 18. E. M. Chellquist, W. G. Reifenrath, E. A. Shipwash, and W. W. Jederberg, Animal models for determining percutaneous absorption of chemicals, Abstract 67, 35th Annum Mtg. Acad. Pharm. Sci., Miami Beach, Florida, November 13-17, 1983. 19. H. Schaefer, A. Zesch, and G. Stuttgen, Skin Permeability, (Springer-Verlag, New York, 1982), pp 548, 601. 20. R. H. Rigdon and A. A. Packchanian, Histologic study of the skin of congenitally athymic (nude) mice, Tex. Rep. on Biol. and Med, 32 (#384), 713-723, 1974. 21. J. B. Wahlberg and G. Swarbeck, The effect of urea and lactic acid on the percutaneous absorption of hydrocortisone, Acta Derre. Venereol. (Stockh), 53, 207-210, 1973. 22. W. Q. Liang and R. V. Petersen, unpublished data.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)