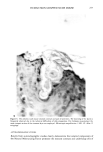
272 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Shampoo Extract a. 7.6 198 5.7 211 100.0 228 1700 15:35 1885 1884 1796 i I I I [ I I I • 1881 1929 I ! 1929 ! I 4 i I I I I I [llllllll'l • 1928 i ] I , , i I ' I [ , I i , , [ I I I"] , [ I [ I [ I I I , i [ i ß . , I 1881 1831 1743 ,, ,,1,8• 1..•928 52 ',, , , ,, ,, ,,q-,,,, ,2•_08, ,, ,2Or__.. 1750 1800 1850 1900 1950 2000 2050 2100 SCAN 16:02 16:30 16:57 17:25 17:52 18:20 18:47 19:15 TIME b. 10.4 198 Shampoo Extract Spiked With NMDDA 100.0 228 1861 I 8 . . ß ' I ' ' ' ' I ' " ' ' [ ' ' ' ' I ' ' ' ' I '[ ' '[ ' I ' ' • [ [ I ] ] 33.8 1862 211 I 1831_• 1.7•.9,.7 1929 ,2q7,2 [ ß ß ' [ .... "• ' ' ' ! ' ' ' ' ! ' •' ' I ' ' ' ' I ' • ' ' I •' ' I 1881 , _ _ _• 1927 ' ' ' ' .... ' ' ' ß ' ' ' ' [ ' "• ' [ ' ' ' ' I ' ' ' ' ] [ [ ' ' 1881 • 19.,.•9_29 2003 2063 I ''' ' ' I .... ! ' ' ' ' ! ' ' ' ' [ " ' ' ' I ' ' ' ' •-' ' ' ' I I I ! I 1700 1750 1800 1850 1900 1950 2000 2050 2100 SCAN 15:35 16:02 16:30 16:57 17:25 17:52 18:20 18:47 19:15 TIME Figure 3. (a) Mass chromatograms for a 0.5 txl splitless injection of a shampoo extract at masses 198 and 211, indicative of NMDDA, and mass 228, the molecular weight of myristic acid. Also shown is the reconstructed ion chromatogram. (b) Mass chromatograms and reconstructed ion chromatogram (as de- scribed above) for a 1 txl splitless injection of the same shampoo extract spiked with 3 txl of a standard 88 ng/txl NMDDA solution. Note the appearance of a new peak at scans 1861/1862.
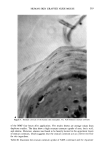
FALSE POSITIVE NITROSAMINE ANALYSES 273 Based upon both the GC/TEA and UV-photolysis experiments, it seemed likely that the compounds responsible for the TEA signal were nitro compounds. Since it was known that the shampoo fragrance contained two nitromusks, musk xylol and mos- kene, they were checked as possible false positives. Again, using the single-ion monitoring capabilities of the GC/MS, the molecular weights ofmoskene (278 amu) and musk xylol (297 amu) were selected along with mass 211 indicative of the NMDDA standard. The same NMDDA spiked shampoo extract shown in Figure 3b is now shown in Figure 4 with single-ion chromatograms at masses 211,278, and 297. Note the appearance of a peak at scan 1888 for mass 297. This peak was easily identified as musk xylol by comparing the background subtracted mass spec- trum of scan 1888 (Figure 5a) to the mass spectrum of a musk xylol standard (Figure 5b). In addition, scan 1928 (mass 278) now clearly stands out in the shampoo extract chromatogram and was identified as moskene also by comparing the unknown spectrum at scan 1928 (Figure 6a) to the mass spectrum of a moskene standard (Figure 6b). A key observation here is the similarity of the retention times of the NMDDA standard at mass 211 (scan 1862) versus the musk xylol in the shampoo extract at mass 297 (scan 1888). These compounds would not be expected to be separated on a non-polar, packed column GC/TEA system. As a final confirmation, both nitromusk PRM were analyzed by GC/TEA and UV-pho- tolysis. As expected, both nitromusks produced a strong signal on the TEA which Shampoo Extract Spiked With NMDDA 100.0 1862 211 I 1831j• • 7,.•7 1929 2072 50.8 278 26.6 297 1928 , l, i i l, , , , i , ,,1,•0.•, , l, ,, , i . . 1,, . , , . i 1888 1852 ,•! 1856 .1881 R IC • 1929 2003 2063 ! ß ß , i'•. • - , , ! 1700 1750 1800 1850 1900 1950 2000 2050 2100 SCAN 15:35 16:02 16:30 16:57 17:25 17:52 18:20 18:47 19:15 TIME Figure 4. Mass chromatograms of the same NMDDA spiked shampoo extract as in Figure 3 at masses 211 (fragment ion of NMDDA), 278 (molecular ion of moskene), and 297 (molecular ion of musk xylol). Also shown is the reconstructed ion chromatogram.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)