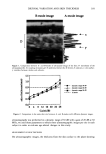
422 JOURNAL OF COSMETIC SCIENCE The wetting data (Figure 2) illustrates this effect'well at HLB 11 (55 wt % EO), all ethoxylates, branched and linear, behave fairly similar with wetting times between 17 and 27 seconds. However, at HLB values 8 or 15 the differences are more significant. A lauryl ethoxylate with HLB 15 has a wetting time of 265 seconds, while a Guerbet 12 Ethoxylate has one of only 34 seconds. In general, Guerbet Ethoxylates are the fastest wetting agents, followed by tridecyl AE and secondary AE while the linear AE are the slowest wetting agents. The foaming test results followed expected trends, more EO provides more foam, a Guerbet 12 AE foams considerably less than linear and tridecyl AE. Cloud Points differ also significantly, which is important for emulsification temperature, since the Cloud Point is essentially the temperature at which the ethoxylate becomes oil soluble. The differences can not be attributed completely to the amount of free alcohol present and must therefore depend on the hydrophobe structure as well. Figures 3 and 4 present the data for critical micelle concentration (CMC) and equilibrium surface tension. These examples show that the surface activity depends greatly on the hydrophobe structure, which complements literature reports on the influence of the alkyl chain length on CMC. 7 While lauryl ethoxylates have the lowest CMC, they are the least effective in lowering the surface tension. Guerbet 12 ethoxylates have the highest CMC, but are very effective in lowering the surface tension. Further investigations regarding micelie shape and size are necessary to explain this phenomenon more clearly. This data helps to determine which surfactant is best for a particular formulation - if the intent for using an ethoxylate is to have a large amount of monomer surfactant present without micellization, the Guerbet ethoxylates should be used. For applications where a small amount of surfactant is meant to yield micelies, the linear lauryl ethoxylates can be recommended. :500- --,- Guerbet 12 AE _ --- Lauryl AE -- -*- Tridecyl AE _ --- Secondary AE • Guerbet 12 AE • --- Lauryl AE --*- Tridecyl AE -..- Secondary AE 45 50 5,5 w•, E060 65 70 75 80 65 70 75 37 E 33 '•' 31 29 27 25 40 45 50 55 • E•O Figure 4. Surface Tension at CMC Figure 3. Critical Micelie Concentration Furthermore, a new set of emulsifiers will be introduced - Guerbet 12 Ethoxylates, Propoxylates and Alkoxylates (EO/PO and PO/EO block polymers), which give the formulator access to a matrix of compatible high and low HLB emulsifiers. They can be used to emulsify a variety of esters and silicone oils. For instance a Guerbet 12 PO/EO Block Polymer will emulsify and thicken a dimethicone, and a Guerbet 12 Propoxylate will dissolve in cyclomethicone and stabilize a water in cyclomethicone emulsion. Solubility and emulsifying behavior of this set of nonionics will be discussed in more detail. These surfactants are liquid over a wider range of temperatures, can improve the skin feel of the formulation and show increased effectiveness as emulsifiers over other surfactants. REFERENCES 1) Cox, M., Weerasooriya, U., J. Surfact. Deterg., 2, 59, (1999). 2) Cox, M., JAOCS, 67, 599 (1990). 3) Cox, M., JAOCS, 66, 367 (1989). 4) Rosen, M. et al., J. Phys. Chem., 86, 541, (1982). 5) Rakatuni, K. et al., "Design and selection of performance surfactants", pp. 216-247, CRC Press (1999). 6) Meerbote, M., Koch, B., Tenside Surf. Det., 31, 39, (1994). 7) J/3nsson, B. et al. "Surfactants and Polymers in Aqueous Solution" John Wiley & Sons (1998).
2001 ANNUAL SCIENTIFIC SEMINAR 423 HURDLE TECHNOLOGY: PRINCIPLES FOR FORMULATING PRESERVATIVE-FREE COSMETICS Jon J. Kabara, Ph.D. Technology Exchange, Inc., PO Box 339, Galena, IL 61036 Introduction Opportunities exist for innovative formulators who want to look beyond current technologies and apply new principles of preservation to the development of novel preservative-free and/or self- preserving products. The application of Hurdle Technology to cosmetic and drug preservation is critical to making such safe and effective products. This technology combines a number of preservative factors (hurdles) that the microorganism(s) in question are not able to survive ("jump over'). Using an array of inhibiting factors (water activity, pH, surfactants, fatty acids and esters, packaging, etc.) allows less extreme use of any particular preservative treatment, especially classical preservatives, to control microbial growth. Such products can be labeled "preservative-free" or more accurately "self-preserving". Water Activity (a,•) • Living organisms have water as an absolute requirement for survival and growth. Limiting the availability of water can usually carry out control of microbial growth. Table 1 shows the lowest values permitting growth of microorganisms important in contaminated cosmetic products. Table 1. Lowest a,• Permitting Growth of Microorganism. Class of Microorganism Minimum a,, Value Pseudomonads 0.97 Coliforms 0.95 Staphylococcus aureus 0.86 Yeasts 0.88 Molds 0.80 Surfactants in Self-Preserving Formulas 2 The primary use of surfactants is as detergents, foaming or wetting agents, solubilizers or dispersants. Surfactants also act as co-emulsifiers because they are partly water soluble and partly oil soluble. This hydrophilic-lipophilic balance (HLB) is a function of specific structure and temperature. Surfactants are classified as anionic, cationic or nonionic. When present in aqueous solution at concentrations above their critical micelie concentration (CMC) surfactants can form micelies. Preservatives absorbed or enclosed in micelies have decreased preservative efficacy due to a reduction in preservative concentration. Surfactants on the other hand may help solubilize preservatives thereby making them more effective. 3 Fatty Acids and Esters as Multifunctional Components Fatty acids have a long history of uses as antimicrobial and anti-insecticidal agents. Monoglycerides which are esters of fatty acids have multifunctioal activity. Highly purified monoglyceride (90%monoester) behaves differently than the usual commercial grade. Distilled monoglycerides form emulsions which are more stable and more easily preserved emulsions. Where the fatty acid is lauric, an added benefit of germicidal action and emolliency has been found.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)