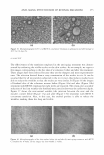
294 JOURNAL OF COSMETIC SCIENCE IN SITU (INTACT) TYROSINE HYDROXYLASE ASSAY Established melanocytes from light and dark skin were subcultivated in six-well plates at a density of 1 x 105 cells/well. Cells were treated daily in triplicate for five days with fresh growth media containing different dosages of test compounds. On the fifth day cultures were assayed for tyrosine hydroxylase activity as previously described (22,23). In short, cells were fed with fresh media containing 1 µCi/ml of H 3 -tyrosine (Amersham Pharmacia Biotech, Piscataway, NJ) and the test compound. After 24-hour incubation, media from each well were collected and diluted in an equal volume of 10% (w/v) activated charcoal in a 0.1 N citric acid solution. Duplicate 1-ml aliquots of the charcoal/ media mixture were passed through a Dowex® 50Wx8-200 acidic cation 1.0-ml ex change column (Sigma-Aldrich, St. Louis, MO) followed by a 1-ml 0.1 N citric acid solution wash. The radioactivity of the tritiated water in the eluate was counted in a Packard 1900 CA liquid scintillation analyzer (Packard Instrument Company, Meriden, CT). The cultured cells in each well were harvested by trypsinization, and the cell suspension was used to determine protein and melanin content. The tyrosinase activity (DPM/24 hours/µg protein) of the cells after treatment was normalized to the activity of control cells. MELANIN AND PROTEIN CONTENT ASSAY Melanin content was determined as previously described (22). In short, cultured cells in each well were harvested with 0.2% trypsin. Cell suspensions were washed twice in phosphate-buffered saline (PBS). The cell pellets were dissolved with 100 µl Triton-X- 100 and lysates centrifuged at 13000 rpm at 4°C for 20 min. The supernatants were used to determine protein content, and pellets were used to determine melanin content. The pellets were washed with 50 µl of ethanol:ether (1:1), lysed in 100 µl of 0.2 N NaOH in 20% DMSO, and the absorbance was measured at 450 nm using a microplate reader (Bio-Rad Model 550, Japan). The melanin content of cells after treatment was expressed as µg melanin/mg protein after normalization with the control. Protein content was determined using the BCA assay (Pierce Chemical, Rockford, IL). In brief, 10 µl of supernatant was added to 200 µl of substrate (50 parts reagent A/1 part reagent B) in a 96-well plate. The plate was incubated for 30 minutes at 3 7°C and then the absorbance was measured at 570 nm in a microplate reader (Bio-Rad Model 550, Japan). The absorbance was compared with a standard curve established using known concentrations of BSA (Pierce Chemical). REVERSIBILITY ASSAY To determine if the effect of dA is reversible, inhibition of tyrosinase act1v1ty and melanin synthesis was assayed in a pulse-chase manner. Established melanocytes from dark skin donors were subcultivated in six-well plates at a density of 1 x 105 cells/well. Experiments were done in triplicate using a previously determined optimal effective dosage of dA that allowed 95 % viability. After five days, a group of dA-treated and vehicle cells were assayed for the inhibition of tyrosinase activity and reduction of melanin content to demonstrate the effect of the test compound. Simultaneously, in another dA-treated group, treatment was halted and this group was fed daily with fresh
EFFICACY AND SAFETY OF DEOXYARBUTIN 295 growth media for an additional five days. At the same time, one of the vehicle-treated group had vehicle treatment halted and this group was fed daily for another five days with fresh growth media. On day 11, the four remaining groups (vehicle-treated for ten days, dA-treated for ten days, dA-treated for five days and then untreated for five days, and vehicle-treated for five days and then untreated for five days) were assessed for tyrosinase activity and melanin content. The same experimental procedure was per formed for HQ and TBP. XENOGRAFTING Xenografts were developed using a protocol approved by the Cincinnati Children's Hospital Medical Center IACUC with animal welfare assurance. Female ICR-SCID mice (Taconic, NY) kept under pathogen-free conditions (Cincinnati Children's Hospital Research Foundation, Cincinnati, OH) were shaved with an electric clipper to remove the dorsal hair. The mice were anesthetized by isofluorane/oxygen (3%/0.8 liter). The dorsal site was cut to produce a wound bed of approximately 2.0-3.0 cm in diameter. Fresh split-thickness cadaveric skin (U.S. Tissues and Cells, Cincinnati, OH) from a Caucasian donor was sutured in place with a reversed cutting precision monofilament PS-3, 6-0 (Moore Medical, CT). Grafts were left untreated for two months, during which time hyperpigmentation occurred. The degree of hyperpigmentation was assessed weekly using a microdigital image obtained from the Charm View™ (Moritex, Japan) surface optical imaging system. The treatment phase was initiated when no further increase in pigmentation was observed. Animals were balanced into three mice per group according to their L values among four treatment groups [deoxyarbutin (dA), hydro quinone (HQ), 4-tertry butyl phenol (TBP), and the vehicle-treated control group}. Treatments were topically applied at a 5 % (w/v) concentration in propylene glycol, ethanol, and water at a volume ration of 1:2:1, at 12.5 µ1/2 cm2 , five days per week, for eight weeks. The treatment sites were assessed on a biweekly basis for the degree of pigmentation using the Charm View™ system. This photographic system took enlarged digital images of the treatment sites, and then with Universal Serial Bus (USB) capture equipment, the images were transferred to a computer. Subsequently, the color param eters for these images (L, a, b) were obtained by using Adobe® Photoshop® software (Adobe Systems Inc., San Jose, Calif.). IMAGE ANALYSIS By using a histogram function in Adobe® Photoshop® software (Adobe Systems Inc.), the color or tonal range of the digital image can be evaluated in either RGB or L, a, b mode. This software allows the desired region of the image to be selected and analyzed. This includes mean, standard deviation, median, and the number of pixels of each color parameter. The L * a* b system is an international standard system, recommended by the CIE (Commission Internationale de I'Eclairage) in 1976 for skin color assessment. In this system, L* a* b color consists of a lightness component (L*), which ranges from Oto 100, and two chromatic components: a* (from green to red) and b* (from blue to yellow).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)