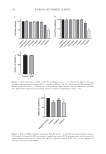
120 JOURNAL OF COSMETIC SCIENCE Figure 3. SEM micrographs of BPO microsponges at different magnifications. Figure 4. Particle size distribution of microsponges. Table II Particle Size and Distribution Values of the Microsponge Dv10 Dv50 Dv90 Span 38.1 µm 111 µm 323 µm 2.567 Figure 5. Fourier transform infrared spectra of pure BPO, EC, and BPO-loaded microsponges.
121 A New Alternative for Acne Treatment The characteristic peaks of BPO and EC can be seen on the infrared spectrum of microsponges with a negligible shift or change. The results indicated that the drug and the polymer had no interaction, and they were compatible with each other. To determine the pore structure of BPO microsponges, nitrogen gas adsorption analysis was performed. The results are given in Table III. The in vitro release from the microsponge for 6 hours is shown in Figure 6. No burst effect has been observed, indicating that BPO was homogeneously dispersed in the microsponge and that no significant amount of the drug was adsorbed onto the microsponge surface. However, as seen from the data obtained after 6 hours, the release rate of formulation was very low (approximately 5%). As the release rate was low, the release study will be continued by changing the dissolution medium. Textile materials that were treated by BPO microsponges were analyzed by SEM. The images are shown in Figure 7. The microparticles preserved their porous surfaces and spherical shapes after the spraying process. The EE of BPO from the fabric samples was found to be 84.193%. The in vitro BPO release from the fabric samples is shown in Figure 8. The data obtained after 3 hours showed that the release rate of formulation was very low (approximately 10%). As the release rate was low, the release study will be continued by changing the dissolution medium. CONCLUSION Within the scope of this study, the potential of microsponges in the textile field was investigated by developing a medical plaster for acne treatment. BPO-loaded plaster can be used on pimples to cover and hide acne. It is also an important advantage to have a Table III Analysis Results of the Microsponge Formulation Surface area Cumulative pore volume Average pore diameter 0.9446 m2/g 0.001017 cm3/g 42.188 Å Figure 6. In vitro drug release of BPO-loaded microsponge.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)