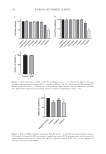
174 JOURNAL OF COSMETIC SCIENCE Robustness. As defined by the ICH, the robustness of an analytical procedure describes its capability to remain unaffected by small and deliberate variations in chromatographic conditions and was studied by testing the influence of small changes in flow rate (mL/ min) and change in mobile phase methanol:water composition (%). No significant effect was observed. The RSD of avobenzone, octinoxate, octocrylene and benzophenone-3 was calculated from the peak area count of five replicate injections, when small but deliberate changes were made to chromatographic conditions. The results are presented in Table V. Thus, the method was found to be robust with respect to variability in chromatographic conditions. Stability of sample solution. The stability of the sample solution was analyzed by storing it at various temperatures. The sample solution was stored under the following conditions: 0.6 h @ RT, 6 h @ 2–8°C, 24 h @ RT, 24 h @ 2–8°C, 30 h @ RT, 30 h @ 2–8°C, 48 h @ RT, and 48 h @ 2–8°C. The assays of avobenzone, octinoxate, octocrylene and benzophenone-3 were analyzed (Table VI). LOD and LOQ. The LOD and LOQ were determined at a signal-to-noise ratio of 3:1 and 10:1 by injecting a series of dilute solutions with known concentrations respectively. The LOD and LOQ values of avobenzone, octinoxate, octocrylene and benzophenone-3 are reported in Table VII. Table IV Recovery Data of Proposed HPLC Method Request No % Recovered (OMC) % Recovered (benzophenone-3) % Recovered (avobenzone) % Recovered (octocrylene) Sunscreen sample A 80% 101.16 108.91 97.07 100.97 Sunscreen sample B 80% 97.75 107.38 94.09 102.12 Sunscreen sample C 80% 98.22 109.17 93.65 99.61 AVERAGE %RSD 99.04 1.86 108.49 0.89 94.94 1.96 100.9 1.76 Sunscreen sample A 100% 104.54 103.71 104.98 111.19 Sunscreen sample B 100% 103.19 101.38 104.51 107.55 Sunscreen sample C 100% 107.05 105.28 108.39 111.47 AVERAGE %RSD 104.93 1.86 103.45 1.89 105.96 1.99 110.07 1.99 Sunscreen sample A 120% 92.26 97.90 85.98 89.85 Sunscreen sample B 120% 93.42 97.81 86.69 88.94 Sunscreen sample C 120% 93.67 101.44 89.27 92.26 AVERAGE %RSD 93.12 0.88 99.05 2.0 87.32 1.98 90.35 1.89 Table V Robustness Data of Proposed HPLC Method Octinoxate Benzophenone-3 Avobenzone Octocrylene Parameter % RSD of STD peak % RSD of assay % RSD of STD peak % RSD of assay Flow rate (0.6 mL) 1.93 1.69 1.69 1.59 Flow rate ( 0.8 mL) 1.84 1.47 1.82 1.5 Mobile phase (85.5 : 14.5) 1.94 1.13 1.9 1.94 Mobile phase ( 87.5 : 12.5) 1.91 1.63 1.8 1.81
175 UV FILTERS IN SUNSCREEN PRODUCTS CONCLUSION An isocratic RP-HPLC method was successfully developed for the estimation of avobenzone, octinoxate, octocrylene, and benzophenone-3 in.sunscreen. The method validation results have proved that the method is selective, precise, accurate, linear, robust, and indicating of stability. We have successfully optimized the method to get a very good separation for octinoxate and avobenzone as they were eluting at very close RT. In addition, the main features of this method are a short run time and all four widely used mentioned UV filters in a single run with a very good separation. The method assessed in the present study met the validation parameters established in the ICH Q2 (R1) validation guidelines (20, 21). This method can be used for the determination of avobenzone, octinoxate, octocrylene, and benzophenone-3 for routine quality control analysis in formulations. REFERENCES (1) A. Salvador and A. Chisvert, Sunscreen analysis, Anal. Chim. Acta, 537(1–2), 1–14 (2005). (2) M. Lorigo and E. Cairrao, Antioxidants as stabilizers of UV filters: an example for the UV-B filter octylmethoxycinnamate, Biomed. Dermatol., 3(1), 11 (2019). (3) N. Duale, A.-K. Olsen, T. Christensen, S. T. Butt, and G. Brunborg, Octyl Methoxycinnamate modulates gene expression and prevents cyclobutane pyrimidine dimer formation but not oxidative DNA damage in UV-exposed human cell lines, Toxicol. Sci., 114(2), 272–284 (2010). (4) A. Nečasová, K. Bányiová, J. Literák, and P. Čupr, New probabilistic risk assessment of ethylhexyl methoxycinnamate: comparing the genotoxic effects of trans- and cis-EHMC, Environ. Toxicol., 32(2), 569–580 (2017). Table VII LOD and LOQ Data of Proposed HPLC Method UV filter LOD (µg/mL) LOQ (µg/mL) S/N Avobenzone 1.88 6.16 4.07 Octinoxate 1.51 4.76 24.82 Octocrylene 2.5 7.82 23.37 Benzophenone-3 1.53 4.75 27.15 Table VI Sample Solution Stability Data of Proposed HPLC Method Sample stability condition % octinoxate % benzophenone-3 % avobenzone % octocrylene Sunscreen sample (Initial/RT) 6.54 5.51 2.49 1.5 Sunscreen sample (6Hr/RT) 6.52 5.44 2.57 1.46 Sunscreen sample (6Hr/2-8°C) 6.29 5.47 2.48 1.53 Sunscreen sample (24Hr/RT) 6.36 5.37 2.54 1.48 Sunscreen sample (24Hr/2-8°C) 6.48 5.40 2.57 1.53 Sunscreen sample (30Hr/RT) 6.39 5.33 2.49 1.52 Sunscreen sample (30Hr/2-8°C) 6.32 5.28 2.5 1.54 Sunscreen sample (48Hr/RT) 6.21 5.43 2.48 1.49 Sunscreen sample (48Hr/2-8°C) 6.35 5.38 2.43 1.47 Sunscreen sample (Initial/RT) 6.54 5.51 2.49 1.5 AVERAGE %RSD 6.38 1.38 5.41 0.98 2.51 1.94 1.53 1.88
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)