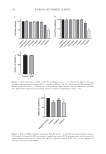
148 JOURNAL OF COSMETIC SCIENCE 5.1 weeks at 40°C and 16.4 weeks at RT. These times were deemed to be undesirable. However, at 40°C, the linear regression of the slope revealed that the buffered version 2.0 (two variants Bd2 and Bi2) at initial pH of 6.2 reduced the rate of pH drift by 5.3 times and 5.5 times, respectively. At 40°C the buffered version 2.55 (20a) reduced the rate of pH drift by 17.7 times. At RT, the buffered versions 2.0 (Bd2 and Bi2) reduced the rate of pH drift by 5.8 times and 4.2 times, respectively. Also, at RT, the buffered version 2.55 (20a) reduced the rate of pH drift by 23.5 times. If the time to arrive at a loss of 1.0 pH unit is selected as one of the criteria for establishing the imputed or formal shelf life, then the sodium citrate:citric acid buffered versions 2.0 (Bd2 and Bi2) can last for at least 1 year prior to a reduction in pH of 1.0 units. Furthermore, version 2.55 (20a) with an initial pH of 5.5 is expected to be stable for multiple years. These results indicate that chemical stability, of which pH is an important marker, has been substantially enhanced by the selected compositions, and especially so at an initial buffered pH of 5.5. The shelf-life estimates may also be established as a function of the concentration of the active ingredient (e.g., IR3535), but the variance should not exceed 5% or 6% loss of the active ingredient over the duration of the imputed or formal shelf life. The concentration of IR3535 of version 2.0 was assessed at 26 weeks at 40°C and RT and was determined to conform to the acceptable variance. Thus, the sodium citrate–citric acid buffered formulation 2.0 (initial pH of 6.2) stabilized not only the pH but also the active ingredient from hydrolysis/degradation. Surprisingly, as previously noted, the formation of a citric acid buffer system by the addition of sodium hydroxide to a citric acid–containing solution did not stabilize the pH the formulations formed by inclusion of trisodium citrate (or the hydrates thereof) were effectively stabilized. EFFICACY OF FORMULATIONS ON HUMANS AGAINST MOSQUITOS IN LABORATORY ARM-IN-CAGE STUDIES The repellent efficacy against mosquitos was assessed for buffered version 2.0 and the unbuffered prototype version 1.0. A laboratory efficacy test (arm-in-cage) was conducted for the version 2.0 on 10 human participants against the mosquito species A aegypti. The dose was 1.0 g of repellent lotion per 600 cm2 applied to the forearm. Table III demonstrates that none of the participants experienced a CFB (i.e., second bite) prior to 13 hours. At 13 hours into the study, 4 of the 10 participants experienced mosquitos landing and/or biting. Thus, the CPT for the protection against the mosquito species Table II Chemical Stability: Elapsed Time (Weeks) to Reach a pH Reduction of 1.0 Unita Version 1.0 - 2.0 (Bd2) 2.0 (Bi2) 2.55 (20a) Buffered pH na 6.2 6.2 5.5 Weeks @ 40°C 5.1 27.2 27.8 90.1 Weeks @ RT 16.4 94.3 68.5 384.6 a Version 1.0 is the unbuffered prototype version 2.0 (Bd2) has ∼2% sodium citrate:citric acid buffer with sodium citrate monohydrate version 2.0 (Bi2) is ∼2% sodium citrate:citric acid buffer with sodium citrate dihydrate version 2.55 (20a) is ∼2% sodium citrate:citric acid buffer and with sodium citrate dihydrate.
149 MICROENCAPSULATED INSECT REPELLENT A aegypti was 13 hours (based upon a CFB criterion i.e., with evidence of a second bite at 14 hours in at least one subject). The unbuffered prototype version 1.0 manifested a CPT of 12 hours using the same experimental method and facility (Table IV). Thus, both microencapsulated formulation version 2.0 and version 1.0 displayed long-duration efficacy, which was superior to the efficacy reported for other commercial products using IR3535 or other active ingredients. Table III Arm-in-Cage Exposure to A aegypti Mosquitos Repellent Version 2.0 Tested on 10 Human Subjects (S1–S10) Hours Version 2.0 Number of bites by A aegypti S1 S2 S3 S4 S5 S6 S7 S8 S9 S10 1 0 0 0 0 0 0 0 0 0 0 2 0 0 0 0 0 0 0 0 0 0 3 0 0 0 0 0 0 0 0 0 0 4 0 0 0 0 0 0 0 0 0 0 5 0 0 0 0 0 0 0 0 0 0 6 0 0 0 0 0 0 0 0 0 0 7 0 0 0 0 0 0 0 0 0 0 8 0 0 0 0 0 0 0 0 0 0 9 0 0 0 0 0 0 0 0 0 0 10 0 0 0 0 0 0 0 0 0 0 11 0 0 0 0 0 0 0 0 0 0 12 0 0 0 0 0 0 0 0 0 0 13 3 0 0 5 0 0 0 2 0 3 14 6 0 0 11 0 0 0 1 0 0 Table IV Arm-in-Cage Exposure to A aegypti Mosquitos Repellent Version 1.0 Tested on 10 Human Subjects (S1–S10) Hours Version 1.0 Number of bites by A aegypti S1 S2 S3 S4 S5 S6 S7 S8 S9 S10 1 0 0 0 0 0 0 0 0 0 0 2 0 0 0 0 0 0 0 0 0 0 3 0 0 0 0 0 0 0 0 0 0 4 0 0 0 0 0 0 0 0 0 0 5 0 0 0 0 0 0 0 0 0 0 6 0 0 0 0 0 0 0 0 0 0 7 0 0 0 0 0 0 0 0 0 0 8 0 0 0 0 0 0 0 0 0 0 9 0 0 0 0 0 0 0 0 0 0 10 0 0 0 0 0 0 0 0 0 0 11 0 0 0 0 0 0 0 0 0 0 12 0 0 1 1 0 0 0 3 0 2 13 0 0 1 8 0 0 0 7 0 5 14
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)