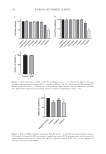
150 JOURNAL OF COSMETIC SCIENCE COSMETIC EXPERIENCE OF HUMANS The lotion versions 2.0 and 2.55 have been applied to the skin of human volunteers for in-life protection against bites from mosquitos and/or flies. The composition versions 2.0 and 2.55 are cosmetically acceptable, quickly dry on the skin, do not have an oily or greasy feel after drying, and do not have an unpleasant aroma (either with or without optional fragrance). Humans have applied the lotions to exposed skin during the daytime and/or at night to protect themselves from mosquitos and/or other biting arthropod pests. ANTI-INFECTIVE PROPERTIES OF COMPOSITIONS CONTAINING IR3535 In Table V, formulation version 2.0 demonstrates antiseptic properties with 60 second exposures against both P aeruginosa and E coli in accordance with the BS EN 1276:2019 test method by achieving a 5 log (base 10) reduction in bacterial load. Version 2.0 also demonstrates some antimicrobial activity against both S aureus and E hirae with a 3 log (base 10) reduction in bacterial load. The results for version 1.0 were slightly better, with 3 of 4 species of microbes exhibiting 5 log killing. Direct comparisons of potency can be made to known topical antiseptics, such as 0.2% sodium hypochlorite (17), 70% ethyl alcohol (albeit at a higher temperature) (18), 70% isopropyl alcohol (19), 3% hydrogen peroxide (19), and 5% tea tree oil (20). Note that versions 2.0 and 1.0 were close to the effectiveness of a common antiseptic, 70% isopropyl alcohol, but they were slightly less effective. The two sustained-release repellent lotions exhibited 99.9% killing. DISCUSSION Chemical stability was a particular challenge of this formulation development effort. The sodium citrate–citric acid buffering achieved chemical stability with regard to pH loss over time and decomposition of IR3535 at both RT and accelerated temperatures (e.g., 40°C). The linear regressions demonstrated that the rate of chemical instability was better ameliorated at an initial pH of 5.5, rather than 6.2, perhaps due to a more optimal buffering capacity with a higher amount of citric acid. Table V Anti-Infective Properties of Repellent Versions 1.0 and 2.0a Disinfectant Log reduction by species (Log10) Effectiveness (%) P aeruginosa E coli S aureus E hirae 0.2% sodium hypochlorite 7 7 7 7 99.9999 70% ethanol 7.7 6.7 6.7 7.4 99.9999 70% isopropanol 5.08 5.37 5.36 5.46 99.999 Version 1.0 5.32 5.39 3.72 5.3 99.9 Version 2.0 5.06 5.26 3.80 3.90 99.9 3% hydrogen peroxide 3.66 2.8 1.32 0.18 90 5% tea tree oil (0.001% tween 80) 1.75 5.25 1 NR 90 a Contact time was 60 seconds. Temperature was 20°C, except for 70% ethanol at an elevated temperature of 34°C.
151 MICROENCAPSULATED INSECT REPELLENT The repellent formulations have an additional benefit as topical consumer products, namely anti-infective properties against four bacterial species in vitro. High potency (i.e., 5 log base 10 order killing) antibacterial effects were observed for 2 or 3 of 4 bacterial strains and moderate potency for the remainder (i.e., 3 log base 10 killing). One may conclude that the 2 versions exhibited 99.9% effectiveness, approaching that of 70% isopropyl alcohol. This anti-infective effect is perhaps due to the preservatives, surfactants (emulsifiers), and/or other excipients known to have anti-infective properties (e.g., acyclic alkane, citric acid, and chitosan), along with perhaps IR3535 (21). Inherent anti-infective properties of a topical repellent formulation may provide coincidental benefits to mammalian skin, such as in the prophylaxis or treatment of infections in compromised skin (abrasions, lacerations, insect bites, etc.). The mosquito repellency efficacy of version 2.0 in human subjects in the laboratory was 13 hours. Thus, this formulation has the hallmarks of a best-in-class repellent. The authors are unaware of any approved commercial repellent product with 12+ hours of protection. An alternative experimental and/or confirmatory approach is to test the efficacy of the topical formulations on humans in life. Human volunteers can be exposed in life to mosquitos, flies, and/or sandflies in outdoor environments known to contain the relevant species of biting insects (22). A common test paradigm is to treat a group of subjects’ exposed skin surfaces with the repellent formulation, and the elapsed time is recorded in 30- or 60-minute intervals until CFB occurs (i.e., when the first individual within a group of subjects experiences a CFB at the time of the second bite). This represents a minimum limit some individuals may or are likely to experience protection beyond the CFB threshold. A preliminary study resembling this type of field trial design was performed with the unbuffered prototype version 1.0 in Florida (United States) and yielded an average protection time of 14 hours (data not shown). Note that an average protection time for a group of subjects can or will be a higher number in hours than a CFB threshold, which is a minimum limits test. It is anticipated that the versions 2.0 and 2.55 will display similar in-life efficacy to that of prototype 1.0, as the unbuffered 1.0 and buffered 2.0 and 2.55 versions all have encapsulated IR3535 at a nominal concentration of 20% (w/w). Regarding repellency of ticks, a pilot study was performed with several human subjects using the prototype version 1.0 at the Arthropod Control Product Test Centre, London School of Hygiene & Tropical Medicine (London, United Kingdom). It yielded an average repellency time of approximately 15–16 hours against Rhipicephalus sanguineus (data not shown). The authors anticipate a similar result with buffered versions 2.0 and 2.55. In aggregate, the preliminary results of the field trial with mosquitos and the pilot study with ticks are suggestive of long-duration repellency, consistent with that reported previously using an arm-in-cage experimental method with mosquitos. Both humans and nonhuman mammals (e.g., horses, cows, dogs, and cats) may benefit from topical application of this formulation. It is expected to be effective immediately, and the duration of effective mosquito repellency may last for 12–13 hours, depending on the species of mosquito, fly, sandfly, black fly, louse, chigger, bed bug, or vector of trypanosomiasis, as well as the noninsect arthropod pest species of tick, chigger (Trombiculidae), spider, or scorpion. The repellent lotion formulation provides a practical method of treating skin, hair, or clothing of a mammal to repel insects or noninsect arthropod pests. Dilutions of the lotion can also be prepared prior to application to the skin, hair, or clothing. For instance, a lotion could be diluted 1:5, 1:10, 1:50, or 1:100 in water or another solution or solvent (e.g., ethanol or ethanol:water). The diluted repellent solution could be applied immediately to
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)